SENSAI CELLULAR PERFORMANCE POWDER FOUNDATION
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATION (REFILL) SPF 15
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- INGREDIENTS
- Warnings
- Direction
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
- PRINCIPAL DISPLAY PANEL - 12g Carton
FULL PRESCRIBING INFORMATION
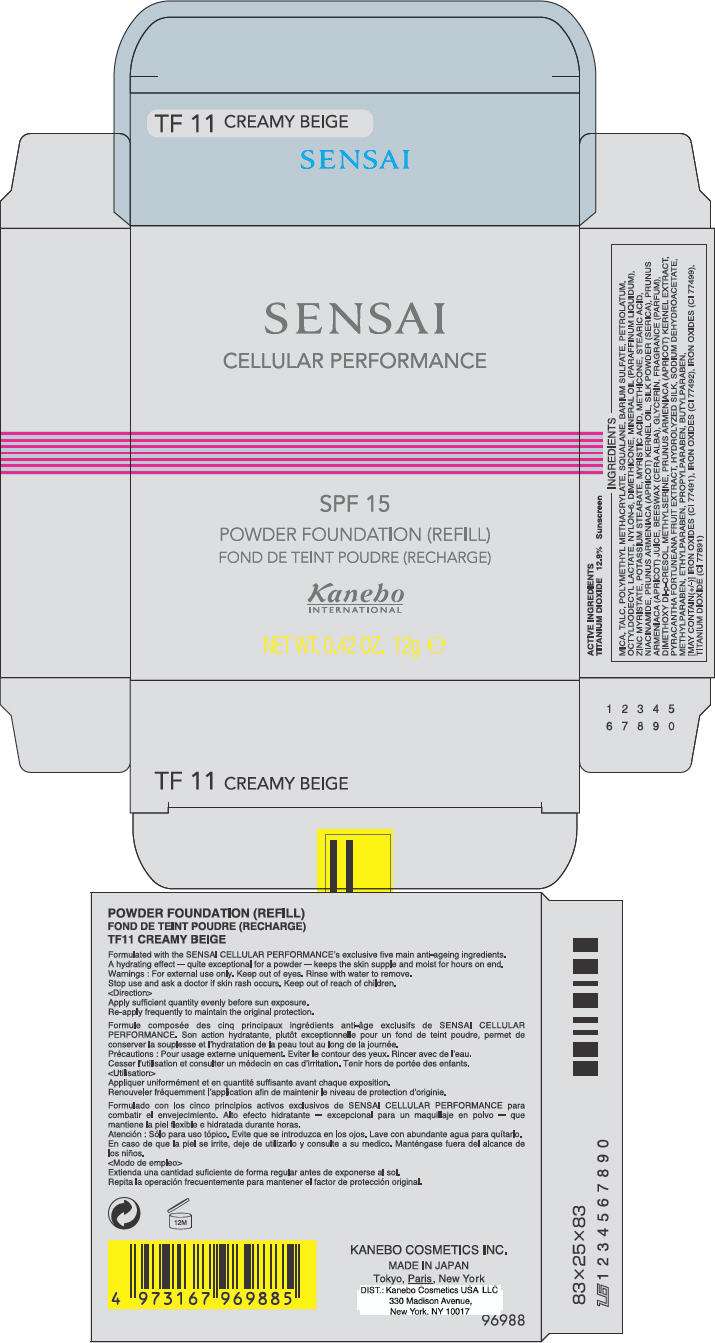
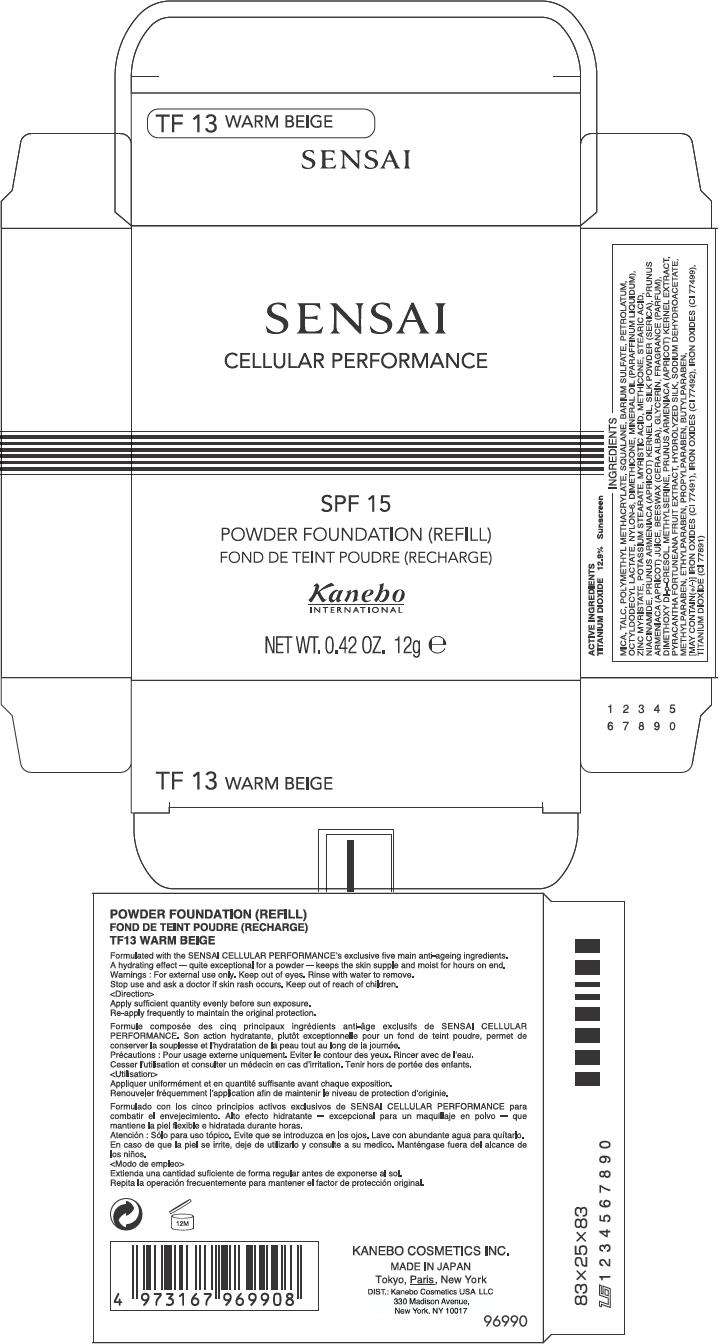
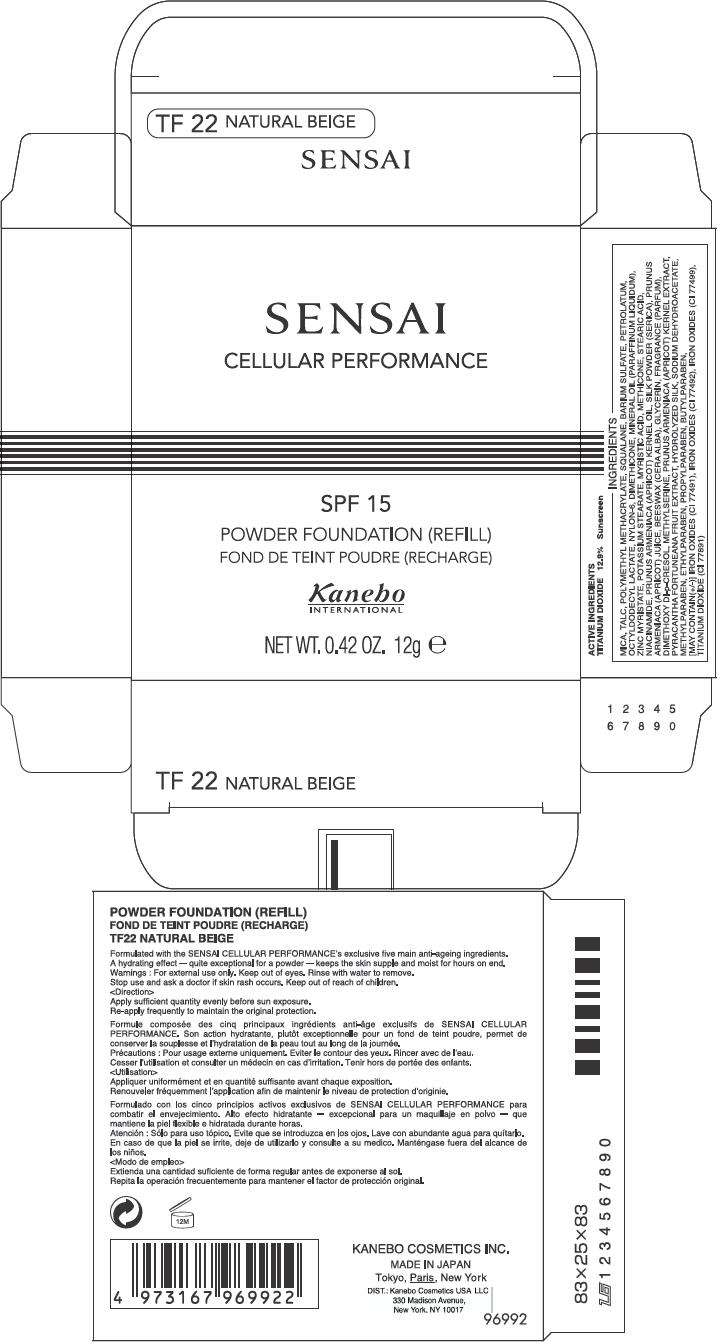
ACTIVE INGREDIENTS
Purpose
TITANIUM DIOXIDE 12.9% Sunscreen
INGREDIENTS
MICA, TALC, POLYMETHYL METHACRYLATE, SQUALANE, BARIUM SULFATE, PETROLATUM, OCTYLDODECYL LACTATE, NYLON-6, DIMETHICONE, MINERAL OIL (PARAFFINUM LIQUIDUM), ZINC MYRISTATE, POTASSIUM STEARATE, MYRISTIC ACID, METHICONE, STEARIC ACID, NIACINAMIDE, PRUNUS ARMENIACA (APRICOT) KERNEL OIL, SILK POWDER (SERICA), PRUNUS ARMENIACA (APRICOT) JUICE, BEESWAX (CERA ALBA), GLYCERIN, FRAGRANCE (PARFUM), DIMETHOXY DI-p-CRESOL, METHYLSERINE, PRUNUS ARMENIACA (APRICOT) KERNEL EXTRACT, PYRACANTHA FORTUNEANA FRUIT EXTRACT, HYDROLYZED SILK, SODIUM DEHYDROACETATE, METHYLPARABEN, ETHYLPARABEN, PROPYLPARABEN, BUTYLPARABEN, [MAY CONTAIN(+/-)] IRON OXIDES (CI 77491), IRON OXIDES (CI 77492), IRON OXIDES (CI 77499), TITANIUM DIOXIDE (CI 77891)
Uses
Formulated with the SENSAI CELLULAR PERFORMANCE's exclusive five main anti-ageing ingredients. A hydrating effect – quite exceptional for a powder – keeps the skin supple and moist for hours on end.
Warnings
For external use only. Keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if skin rash occurs. Keep out of reach of children.
Direction
Apply sufficient quantity evenly before sun exposure.
Re-apply frequently to maintain the original protection.
DIST.: KANEBO COSMETICS USA LLC
330 Madison Avenue, New York, NY 10017
PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e
PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e

PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e
PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e
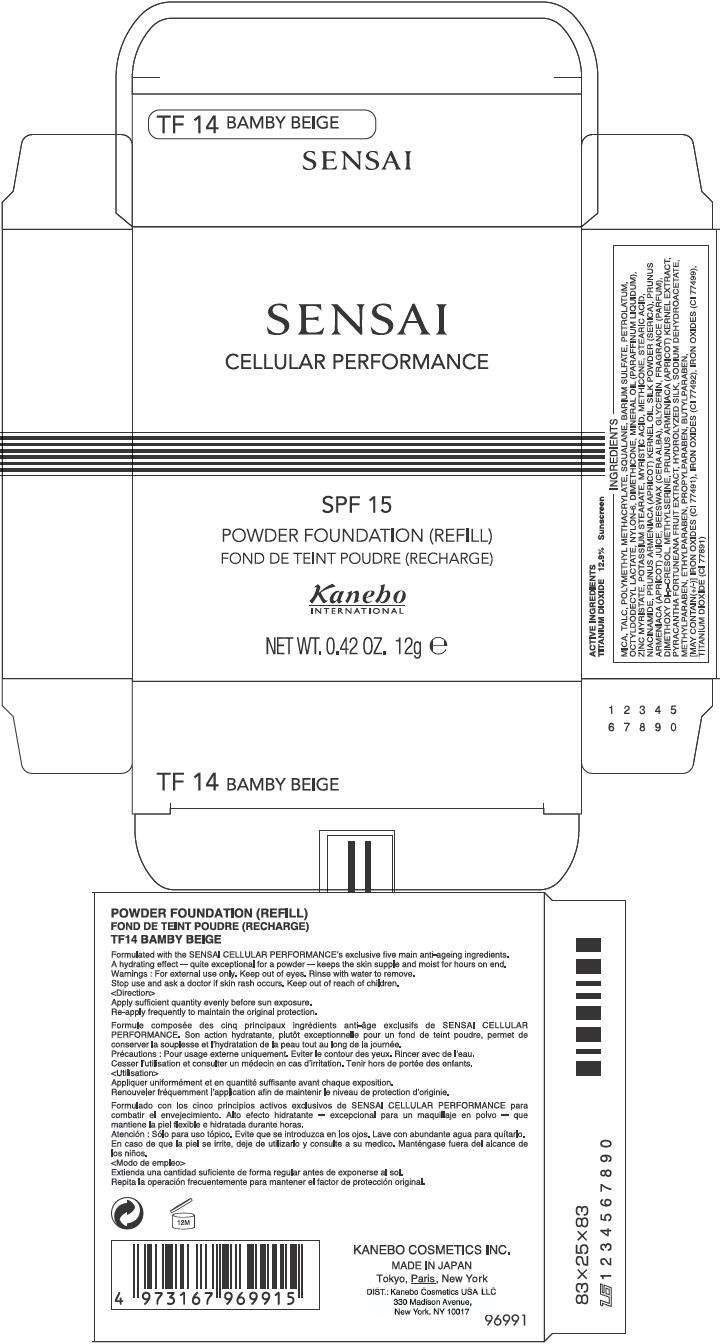
PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e
PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e

PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e

PRINCIPAL DISPLAY PANEL - 12g Carton
SENSAI
CELLULAR PERFORMANCE
SPF 15
POWDER FOUNDATION (REFILL)
Kanebo
INTERNATIONAL
NET WT. 0.42 OZ. 12g e

SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||
SENSAI CELLULAR PERFORMANCE POWDER FOUNDATIONTITANIUM DIOXIDE POWDER
| |||||||||||||||||||||||||||||||||||||||||||||||||