SEBASORB
Summers Laboratories Inc
FULL PRESCRIBING INFORMATION
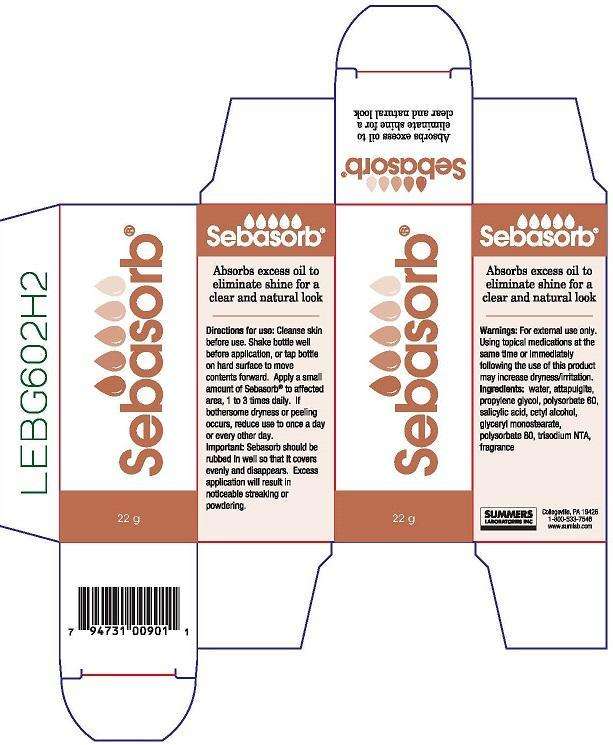
Product Information
|
|
Product Type
|
Cosmetic |
Item Code (Source)
|
NDC:11086-009 |
|
Route of Administration
|
|
DEA Schedule
|
|
Packaging
|
|
#
|
Item Code
|
Package Description
|
Marketing Start Date
|
Marketing End Date
|
|
1 |
NDC:11086-009-01 |
22 in 1 BOTTLE |
|
|
|
2 |
NDC:11086-009-11 |
1 in 1 BOX |
|
|
Marketing Information
|
|
Marketing Category
|
Application Number or Monograph Citation
|
Marketing Start Date
|
Marketing End Date
|
|
|
|
2013-10-29 |
|
|
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!

