Scott Clear Antibacterial Skin Cleanser
Scott Clear Antibacterial Skin Cleanser
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active Ingredient
- Purpose
- Use
- Warnings
- Directions
- Other Information
- Inactive Ingredients
- Questions?
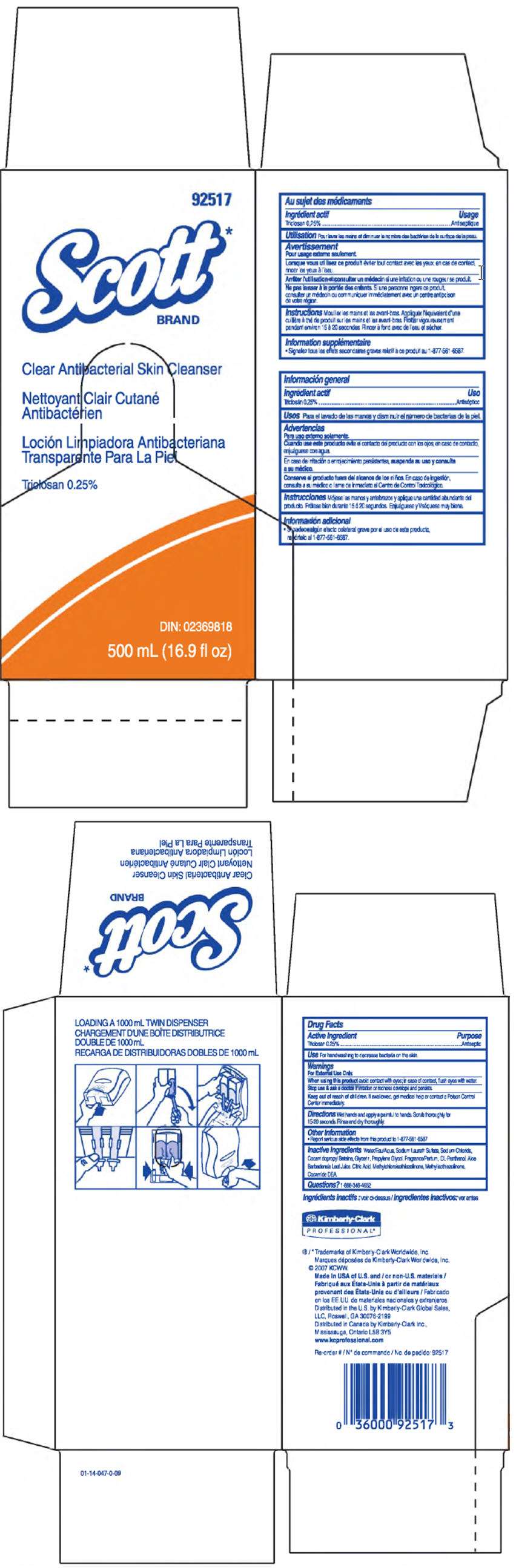
- PRINCIPAL DISPLAY PANEL - 500 mL Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active Ingredient
Triclosan 0.25%
Purpose
Antiseptic
Use
For handwashing to decrease bacteria on the skin.
Warnings
For External Use Only.
When using this product avoid contact with eyes; in case of contact, flush eyes with water.
Stop use & ask a doctor if irritation or redness develops and persists.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center immediately.
Directions
Wet hands and apply a palmful to hands. Scrub thoroughly for 15-20 seconds. Rinse and dry thoroughly.
Other Information
- Report serious side effects from this product to 1-877-561-6587
Inactive Ingredients
Water, Sodium Laureth Sulfate, Sodium Chloride, Cocamidopropyl Beteine, Glycerin, Propylene Glycol, Frangrance, DL-Panthenol, Aloe Barbadensis Leaf Juice, Citric Acid, Methylchloroisothiazolinone, Methylisothiazolinone, Cocamide DEA.
Questions?
1-888-346-4652
Distributed in the U.S. by Kimberly-Clark Global Sales, LLC, Roswell, GA 30076-2199
Distributed in Canada by Kimberly-Clark Inc., Mississauga, Ontario L5B 3Y5
PRINCIPAL DISPLAY PANEL - 500 mL Carton
92517
Scott*
BRAND
Clear Antibacterial Skin Cleanser
Triclosan 0.25%
DIN: 02369818
500 mL (16.9 fl oz)
Scott Clear Antibacterial Skin CleanserTriclosan SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||