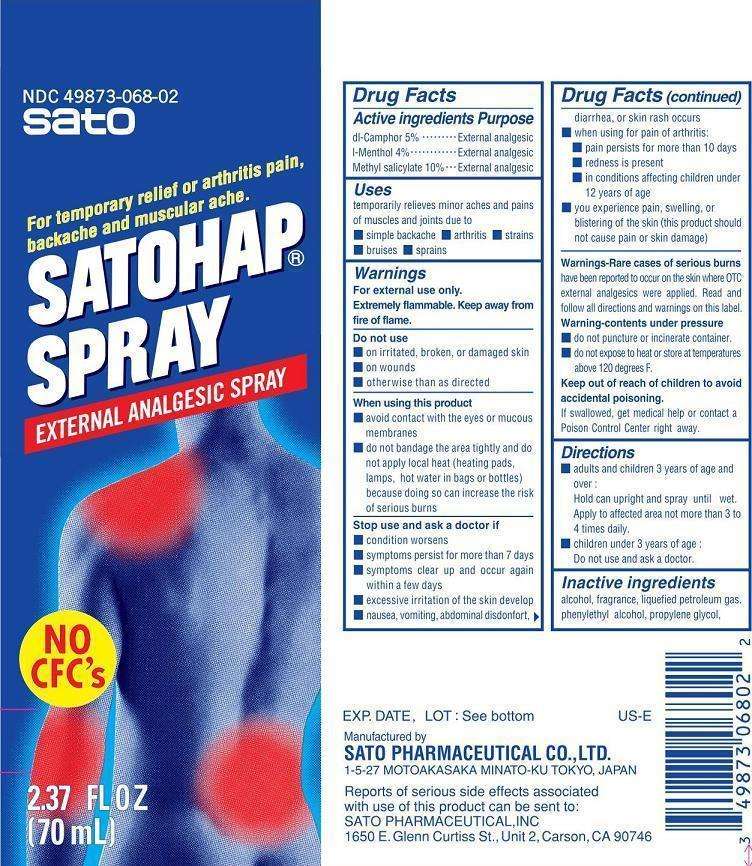
Satohap
Drug Facts
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredients
dl-Camphor 5%
l-Menthol 4%
Methyl salicylate 10%
Purpose
Purpose
dl-Camphor External analgesic
l-Menthol External analgesic
Methyl salicylate External analgesic
Uses
Uses temporarily relieves minor aches and pains of muscles and joints due to
- simple backache
- arthritis
- strains
- bruises
- sprains
Warnings
For external use only.
Extremely flammable. Keep away from fire or flame.
Do not use
- on irritated, broken, or damaged skin
- on wounds
- otherwise than as directed
When using this product
- avoid contact with the eyes or mucous membranes
- do not bandage the area tightly and do not apply local heat (heating pads, lamps, hot water in bags or bottles) because doing so can increase the risk of serious burns
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- symptoms clear up and occur again within a few days
- excessive irritation of the skin develops
- nausea, vomiting, abdominal discomfort, diarrhea, or skin rash occurs
- when using for pain of arthritis:
pain persists for more than 10 days
redness is present
in conditions affecting children under 12 years of age
- you experience pain, swelling, or blistering of the skin (this product should not cause pain or skin damage)
Warning-Rare cases of serious burns have been reported to occur on the skin where OTC external analgesics weere applied. Read and follow all directions and warnings on this label.
Warning-contents under pressure
- do not puncture or incinerate container
- do not expose to heat or store at temperatures above 120 degrees F.
Keep out of reach of children to avoid accidental poisoning. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 3 years of age and over: Hold can upright and spray until wet. Apply to affected area not more than 3 to 4 times daily.
- children under 3 years of age: Do not use and ask a doctor.
Inactive ingredients
alcohol, fragrance, liquefied petroleum gas, phenylethyl alcohol, propylene glycol

Satohapmethyl salicylate, dl-camphor, l-menthol SPRAY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||