Salvax
Quinnova Pharmaceuticals, Inc.
Hydrating Topical Foam(salicylic acid in a water and lipid based foam, 6%)
FULL PRESCRIBING INFORMATION: CONTENTS*
- SALVAX DESCRIPTION
- CLINICAL PHARMACOLOGY
- SALVAX INDICATIONS AND USAGE
- SALVAX CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- SALVAX ADVERSE REACTIONS
- SALVAX DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
SALVAX DESCRIPTION
SALVAX is applied topically and used in the removal of excessive keratin in hyperkeratotic skin disorders. Each gram of SALVAX contains salicylic acid 6% as the active ingredient, and the following inactive ingredients: dimethicone, ethylparaben, glycerin, methylcellulose, methylparaben, phenoxyethanol, polyoxyl 40 stearate, polysorbate 20, polysorbate 80, povidone, propylene glycol, propylparaben, purified water, sodium citrate, sodium hydroxide, stearic acid, and trolamine and in propellants butane and propane.
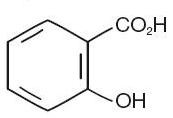
CHEMICAL STRUCTURE
Salicylic acid is the 2-hydroxy derivative of benzoic acid having the following chemical structure:
CLINICAL PHARMACOLOGY
Salicylic acid has been shown to produce desquamation of the horny layer of skin while not affecting qualitative or quantitative changes in structure of the viable epidermis. The mechanism of action has been attributed to dissolution of intercellular cement substance. In a study of the percutaneous absorption of salicylic acidfrom SALVAX infour patients with extensive active psoriasis, Taylor and Halprin showed that peak serum salicylate levels never exceeded 5 mg/100 ml even though more than 60% of the applied salicylic acid was absorbed. Systemic toxic reactions are usually associated with much higher serum levels(30 to 40mg/100ml). Peak serum levels occurred within 5 hours of the topical application under occlusion. The sites were occluded for 10 hours over the entire body surface below the neck. Since salicylates are distributed in the extracellular space, patients with a contracted extracellular space due to dehydration or diuretics have higher salicylate levels than those with a normal extracellular space. (See PRECAUTIONS).
The major metabolites identified in the urine after topical administration are salicyluric acid (52%), salicylate glucuronides (42%), and free salicylic acid (6%). The urinary metabolites after percutaneous absorption differ from those after oral salicylate administration; those derived from percutaneous absorption contain more glucuronides and less salicyluric and salicylic acid. Almost 95% of a single dose of salicylate is excreted within 24 hours of its entrance into the extracellular space.
Fifty to eighty percent of salicylate is protein bound to albumin. Salicylates compete with the binding of several drugs and can modify the action of these drugs. By similar competitive mechanisms other drugs can influence the serum levels of salicylate. (See PRECAUTIONS).
PHARMACOKINETICS
The mechanism of action of topically applied salicylic acid has been attributed to the dissolution of intercellular cement substance.
SALVAX INDICATIONS AND USAGE
For Dermatologic Use: SALVAX is a topical aid in the removal of excessive keratin in hyperkeratotic skin disorders, including verrucae and the various ichthyoses, keratosis palmaris and plantaris, keratosis pilaris, pityriasis rubra pilaris, and psoriasis.
For Podiatric Use: SALVAX is a topical aid in the removal of excessive keratin on dorsal and plantar hyperkeratotic lesions.
SALVAX CONTRAINDICATIONS
SALVAX should not be used in any patient known to be sensitive to salicylic acid or any other listed ingredients. SALVAX should not be used in children under 2 years of age.
WARNINGS
SALVAX is for external use only. It is not for ophthalmic, oral, anal or intravaginal use. Contact with eyes, lips, broken or inflamed skin, and all mucous membranes should be avoided. SALVAX should not be used by persons who have a known hypersensitivity to salicylic acid or any of the other listed ingredients.
Prolonged use over large areas, especially in children and those patients with significant renal or hepatic impairment could result in salicylism. Concomitant use of other drugs which may contribute to elevated serum salicylate levels should be avoided where the potential for toxicity is present. In children under 12 years of age and those patients with renal or hepatic impairment, the area to be treated should be limited and the patient monitored closely for signs of salicylate toxicity: nausea, vomiting, dizziness, loss of hearing, tinnitus, lethargy, hyperpnoea, diarrhea, psychic disturbances. In the event of salicylic acid toxicity, the use of SALVAX should be discontinued. Fluids should be administered to promote urinary excretion. Treatment with sodium bicarbonate (oral or intravenous) should be instituted as appropriate.
Considering the potential risk of developing Reye’s syndrome, salicylate products should not be administered to children or teenagers with varicella or influenza, unless directed by a physician.
PRECAUTIONS
SALVAX should be used only as directed by a physician and should not be used to treat any condition other than that for which it is prescribed. SALVAX should not be used on any skin area where inflammation or exudation is present as increased absorption may occur. If redness or irritation occurs, discontinue use and consult with prescribing physician.
I. Due to the competition of salicylate with other drugs forbinding to serum albumin the following drug interactions may occur:
| Drug | Description of Interaction |
| Tolbutamide; Sulfonylureas | Hypoglycemia potentiated |
| Methotrexate | Decreases tubular reabsorption; clinical toxicity from methotrexate can result |
| Oral Anticoagulants | Increased Bleeding |
II. Drugs changing salicylate levels by altering renal tubular reabsorption:
| Drug | Description of Interaction |
| Corticosteroids | Decreases plasma salicylate level; tapering doses of steroids may promote salicylism |
| Ammonium Sulfate |
Increases plasma salicylate level |
III. Drugs with complicated interactions with salicylates:
| Drug | Description of Interaction |
| Heparin | Salicylate decreases platelet adhesiveness and interferes with hemostasis in heparin-treated patients |
| Pyrazinamide | Inhibits pyrazinamide-induced hyperuricemia |
| Uricosuric Agents | Effect of probenecid, sulfinpyrazone and phenylbutazone inhibited |
The following alterations of laboratory tests have been reported during salicylate therapy:
| Laboratory Tests | Effects of Salicylates |
| Thyroid Function | Decreased PBI; increased T3 uptake |
| Urinary Sugar | False negative with glucose oxidase; false positive with Clinitest with highdose salicylate therapy (2-5 g qd) |
| 5 Hydroxyindole AceticAcid | False negative with fluorometric test |
| Acetone, Ketone Bodies | False positive FeCl3 inGerhardt reaction; red color persists with boiling |
| 17-OH Corticosteroids | False reduced values with >4.8 g qd salicylate |
| Vanilmandelic Acid | False reduced values |
| Uric Acid | May increase or decrease depending on dose |
| Prothrombin | Decreased levels; slightly increased prothrombin time |
Salicylic acid has been shown to be teratogenic in rats and monkeys. It is difficult to extrapolate from oral doses of acetylsalicylic acid used in these studies to topical administration as the oral dose to monkeys may represent 4 times the maximum daily human dose of salicylic acid (as supplied in one tube, 40 g of SALVAX) when applied topically over a large body surface. There are no adequate and well controlled studies in pregnant women. SALVAX should be used during pregnancy only if the potential benefit justifies the risk to the fetus.
It is not known whether topically applied salicylic acid is excreted in human milk. Due to the fact that many drugs are excreted in human milk, caution should be exercised by physicians when administering SALVAX to nursing mothers and nursing mothers should certainly not apply SALVAX to the chest area or any other part of the body with which the nursing child’s mouth is likely to come in contact.
Because of the potential for serious adverse reactions in nursing infants from the mother’s use of SALVAX, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
No data are available concerning potential carcinogenic or reproductive effects of SALVAX. It has been shown to lack mutagenic potential in the Ames Salmonella test.
KEEP THIS AND ALL OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
SALVAX ADVERSE REACTIONS
Transient stinging, burning, itching or irritation is possible. Peeling of the skin may increase as the salicylic acid works to loosen excess keratin. If excessive burning, stinging or peeling occurs, discontinue use and consult your physician.
DOSAGE - See WARNINGS
SALVAX DOSAGE AND ADMINISTRATION
Unless otherwise directed by a prescribing physician, SALVAX should be applied to affected area twice a day. SALVAX should be rubbed into the skin until it is completely absorbed.
SALVAX should be shaken vigorously before each application and inverted to administer.
HOW SUPPLIED
SALVAX is supplied in a 70 gram or 2.5 ounce aerosolized canister bearing the NDC Number 23710-006-70, a 200 gram or 7.1 ounce aerosolized canister bearing the NDC Number 23710-006-02, a 22 gram or 0.79 ounce aerosolized canister bearing the NDC Number 23710-006-20, and a 10 gram or 0.36 ounce aerosolized canister bearing the NDC Number 23710-006-01. The 10 gram canister is a physician dispensed sample product.
Store at controlled room temperature 15° - 25°C (59° - 77°F).
Contains flammable materials. Contents under pressure. Do not puncture and/or incinerate the containers. Do not expose to temperatures over 120°F (48°C) even when empty.
U.S. PATENT PENDING FOR SALVAX.
SALVAX is manufactured for Quinnova Pharmaceuticals, Inc., Newtown, PA 18940, (877) 660-6263, www.QUINNOVA.com
Prescribing Information as of April 2009.
SALVX014 4/09



Salvaxsalicylic acid AEROSOL, FOAM
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||