Salicylic Acid 6 percent
SALICYLIC ACID SHAMPOO
FULL PRESCRIBING INFORMATION
Salicylic Acid Shampoo contains 6% w/w salicylic acid USP in a
vehicle consisting of purified water, acrylates copolymer, sodium
laureth sulfate, trolamine, quaternium 26 and propylene glycol,
cocamidopropyl betaine, behentrimonium methosulfate and
cetearyl alcohol, propylparaben, methylparaben, glycerin, disodium
EDTA and chamomile tea fragrance.
Salicylic acid is the 2-hydroxy derivative of benzoic acid having
the following structure:
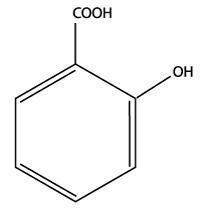
Uses
Salicylic Acid 6% (w/w) Shampoo should not be used in any
Patient known to be sensitive to salicylic acid or any other listed
ingredients. Salicylic Acid 6% (w/w) Shampoo should not be
used in children under 2 years of age.
The following interactions are from a published review and
include reports concerning both oral and topical salicylate admin-
istration. The relationship of these interactions to the use of
Salicylic Acid 6% (w/w) Shampoo is not known.
I.Due to the competition of salicylate with other drugs for
bindingto serum albumin the following drug interactions may
occur:
DRUGDESCRIPTION OF INTERACTION
SulfonylureasHypoglycemia potentiated.
MethotrexateDecreases tubular reabsorption; clinical
toxicity from methotrexate can result.
OralIncreased bleeding.
Anticoagulants
II.Drugs changing salicylate levels by altering renal tubular
reabsorption:
DRUGDESCRIPTION OF INTERACTION
CorticosteroidsDecreases plasma salicylate level; tapering
doses of steroids may promote salicylism.
AcidifyingIncreases plasma salicylate level.
Agents
AlkanizingDecreased plasma salicylate levels.
Agents
III.Drugs with complicated interactions with salicylates:
DRUGDESCRIPTION OF INTERACTION
HeparinSalicylate decreases platelet adhesiveness
and interferes with hemostasis in heparin
treated patients.
Pyrazinamide
Inhibits pyrazinamide-induced
uricemia.
hyper-
UricosuricEffect of probenemide, sulfinpyrazone and
phenylbutazone inhibited.
The following alterations of laboratory tests have been reported
during salicylate therapy:
LABORATORYEFFECT OF SALICYLATES
TESTS
ThyroidDecreased PBI; increased Tuptake.
3
Function
Urinary SugarFalse negative with glucose oxidase; false
positive with Clinitest with high-dose sali-
cylate therapy (2-5g q.d.).
5- HydroxyindoleFalse negative with fluorometric test.
acetic acid
Acetone,False positive FeClin Gerhardt reaction;
3
ketone bodiesred color persists with boiling.
17-OHFalse reduced values with >4.8g
corticosteroidsq.d. salicylate.
VanilmandelicFalse reduced values.
acid09-0080
Uric acidMay increase or decrease depending on
dose.
ProthrombinDecreased levels; slightly Increased pro-
thrombin time.
Because of the potential for serious adverse
reactions in nursing infants from the mother’s use of Salicylic
Acid 6% (w/w) Shampoo, a decision should be made whether to
discontinue nursing or to discontinue the drug, taking into
account the importance of the drug to the mother. If used by
nursing mothers, it should not be used on the chest area to avoid
the accidental contamination of the child.
No data are available concerning potential carcinogenic or reproductive
effects of Salicylic Acid 6% (w/w) Shampoo. Salicylic acid has
been shown to lack mutagenic potential in the Ames Salmonella
test.
Excessive erythema and scaling conceivably could result from
use on open skin lesions.
See Warnings.
Salicylic Acid 6% (w/w) Shampoo is available in 177mL plastic
bottles (NDC 42546-279-06).
Store at controlled room temperature 20°-25°C (68°-77°F). Do
not freeze
NDC 42546-279-06
Rx Only
Salicylic Acid Shampoo 6% (w/w)
177mL
PruGen, Inc. Pharmaceuticals

Salicylic Acid 6 percentSalicylic Acid SHAMPOO
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||