Ryneze Liquid
SJ PHARMACEUTICALS, LLC
Great Southern Laboratories
Ryneze Liquid
FULL PRESCRIBING INFORMATION: CONTENTS*
- RYNEZE LIQUID DESCRIPTION:
- CLINICAL PHARMACOLOGY:
- RYNEZE LIQUID INDICATIONS AND USAGE:
- RYNEZE LIQUID CONTRAINDICATIONS:
- WARNINGS:
- PRECAUTIONS:
- Information for patients:
- Laboratory Tests:
- Drug Interactions:
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- Pregnancy:
- Nursing Mothers:
- Pediatric Use:
- Geriatric Use:
- RYNEZE LIQUID ADVERSE REACTIONS:
- OVERDOSAGE:
- RYNEZE LIQUID DOSAGE AND ADMINISTRATION:
- HOW SUPPLIED:
- PRODUCT PACKAGING:
FULL PRESCRIBING INFORMATION
DESCRIPTION:
Each
teaspoonful (5 mL) for oral administration
contains:
Chlorpheniramine
Maleate ...................................... 4 mg
Scopolamine
Methyl Nitrate .............................. 1.25 mg
INACTIVE
INGREDIENTS: Citric Acid, Grape flavor, Glycerin, Propylene
Glycol, Purified Water, Sodium Citrate, Sodium
Saccharin,
and Sorbitol.
RYNEZE® LIQUID contains ingredients from the following classes: antihistamine, and anticholinergic.
Chlorpheniramine
Maleate is an antihistamine with the chemical
name: 2-Pyridinepropanamine, γ-(4-chlorphenyl)-
N,N-dimethyl-,
(Z)-2-butenedioate (1:1), and has the following
chemical structure:
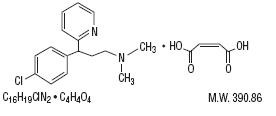
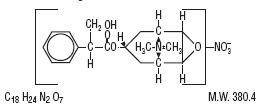
Scopolamine
Methyl Nitrate is an anticholinergic belldonna alkaloid
derivative with the chemical name:
[7(s)-(α
,2β,4β,5α,7β,)]–7–(3–hydroxy–1-oxo – 2- Phenylpropoxy)-9,
9-dimethyl–3-oxa– 9-azoniatricyclo
[3.3.1.0
2,4] nonane nitrate.It
has the following chemical structure:
CLINICAL PHARMACOLOGY:
Chlorpheniramine
maleate competitively antagonizes most of the
smooth muscle stimulating actions of histamine on the
H1
receptors of the GI tract, uterus, large blood vessels, and bronchial
muscle. It also antagonizes the action of histamine
that
results in increased capillary permeability and the formation
of edema. Chlorpheniramine maleate is analkylamine-type
antihistamine.
This group of antihistamines are
among the most active histamine antagonists and are
generally
effective in relatively low doses. They thereby prevent,
but do not reverse, responses mediated by histamine
alone.
The anticholinergic actions of most antihistamines provide
a drying effect on the nasal mucosa. These drugs are
not
so prone to produce drowsiness and are among the most suitable
agents for daytime use, but a significant proportion
of
patients do experience this effect.
Scopolamine
methyl nitrate is one of the principal anticholinergic/antispasmodic
components of belladonna
alkaloids
that exhibits antisecretory activity. Scopolamine methyl
nitrate inhibits the muscarinic actions of acetylcholine
on
structures innervated by postganglionic cholinergic nerves:
smooth muscle, cardiac muscle, sinoatrial and
atrioventricular
nodes, and exocrine glands. In general, the smaller
doses of anticholinergics inhibit salivary and
bronchial
secretions, sweating, and accommodation; cause dilation
of the pupil; and may affect the heart rate.
INDICATIONS AND USAGE:
This
product is indicated for the temporary
relief of symptoms associated with seasonal and
perennial
allergic and non-allergic rhinitis, and sinusitis.Chlorpheniramine
Maleate temporarily relieves runny nose
and
reduces sneezing, itching of the nose or throat, and itchy,watery
eyes due to hay fever or other upper respiratory
allergies.
Scopolamine Methyl Nitrate further augments the anti-secretory
activity of this product.
CONTRAINDICATIONS:
This
product is contraindicated in patients
with hypersensitivity or idiosyncrasy to any of its
ingredients.
It is also contraindicated in women who are pregnant
or nursing.
It is
also contraindicated in newborn or premature infants, because
this age group has an increased susceptibility to the
anticholinergic
side effects of chlorpheniramine maleate. Geriatric patients
may be more sensitive to the effects of this medication.
Risk-benefit
should be considered when the following conditions
exist: Acute asthma; Bladder neck obstruction;
Brain
damage in children; Cardiac disease, especially cardiac arrhythmias,
congestive heart failure, coronary artery disease,
and
mitral stenosis; Cardiovascular disease; Diabetes mellitus;
Down’s Syndrome; Esophagitis, reflux; Narrow angle
glaucoma;
Acute hemorrhage with unstable cardiovascular status;
Hepatic function impairment; Hernia; Hypertension;
Hyperthyroidism;
Intestinal atony in the elderly or debilitated patient;
Chronic lung disease; Myasthenia gravis; Autonomic
neuropathy;
Paralytic ileus; Prostatic hypertrophy; Psychiatric disorders;
Pyloric obstruction; Renal function impairment;
Spastic
paralysis, in children; Tachycardia; Toxemia of pregnancy;
Ulcerative colitis; Urinary retention, or
predisposition
to; Uropathy; Xerostomia.
WARNINGS:
This
product may cause drowsiness or blurred vision.
Patients taking this product should be warned not to
engage
in activities requiring mental alertness such as operating
a motor vehicle or other machinery or to perform
hazardous
tasks while taking this drug.
Do not exceed recommended dosage.
Heat
prostration can occur with the use of scopolamine methyl
nitrate when the environmental temperature is high.
Diarrhea
may be an early symptom of incomplete intestinal obstruction,
especially in patients with ileostomy or
colostomy;
in this instance, use of scopolamine methyl nitrate would
be inappropriate and possibly harmful.
PRECAUTIONS:
General:
Antihistamines have an atropine-like
action and should be used with caution in
patients
with a history of bronchial asthma, emphysema, increased
intraocular pressure, hyperthyroidism,
cardiovascular
disease and hypertension.
Use
scopolamine methyl nitrate with caution in patients with
hiatal hernia associated with reflux Esophagitis. Use
extreme
caution and only when needed in patients with autonomic
neuropathy, hyperthyroidism, coronary heart
disease,
congestive heart failure, and cardiac arrhythmia.
Information for patients:
Patient
consultation should include the following information
regarding proper use of this medication:
• Do
not take more medication than the amount recommended.
•
This medication should be used with caution during exercise
or hot weather, overheating may result in heat
stroke.
• Do
not drive or operate machinery if drowsiness or dizziness
occurs.
• Do
not ingest alcoholic beverages, monoamine oxidase
(MAO) inhibitors, or CNS depression producing
medications
(hypnotics, sedatives, tranquilizers)
while taking this medication.
•
This medication possibly increases sensitivity of eyes to
light.
•
Scopolamine methyl nitrate may cause blurred vision.
• If
a dose is missed, the medication should be taken as soon
as possible unless it is almost time for the next
dose.
Do not double dose.
Caution
patients about the signs of potential side effects,
especially:
•
Anticholinergic effects – clumsiness or unsteadiness;severe
drowsiness; severe dryness of mouth, nose, or
throat;
flushing or redness of face; shortness of breath or
trouble breathing.
•
Blood dyscrasias-sore throat and fever; unusual bleeding
or bruising; unusual tiredness or weakness.
•
Fast or irregular heartbeat.
•
Psychotic episodes.
•
Tightness in chest.
Note:
When anticholinergics are given to patients, especially
children, where the environmental temperature
is
high there is a risk of a rapid increase in body temperature
because of suppression of sweat gland
activity.
Infants, patients with Down’s syndrome, and children
with spastic paralysis or brain damage may show
an
increased response to anticholinergics, thus increasing the
potential for side effects.
Geriatric
or debilitated patients may respond to usual doses
of anticholinergics with excitement, agitation,
drowsiness,
or confusion.
Laboratory Tests:
The
following may be especially important
in patient monitoring (other tests may be
warranted
in some patients, depending on conditions): Blood
pressure determination – recommended at
frequent
intervals during therapy: Electrocardiogram (ECG)
– monitoring may be required: Intraocular
pressure
determination – recommended at periodic intervals,
as these medications may increase the
intraocular
pressure.
Drug Interactions:
Do
not take this product if you are presently
taking, or have taken within the preceding
two
weeks, a prescription drug for high blood pressure or
depression without first consulting your physician.
Absorption
of other oral medications may be decreased
during concurrent use with anticholinergics
due
to decreased gastrointestinal motility and delayed gastric
emptying.
Combinations
containing any of the following medications,
depending on the amount present, may
also
interact with this product:
•
Alkalizers, such as: calcium and/or magnesium-containing
antacids; Carbonic inhibitors;
citrates;
sodium bicarbonate-urinary excretion of anticholinergics
may be delayed by alkalization of the
urine,
thus potentiating scopolamine methyl nitrate therapeutic
and/or side effects.
•
Antacids or adsorbent antidiarrheals-simultaneous use
of these medications may reduce absorption of
scopolamine
methyl nitrate, resulting in decreased therapeutic
effectiveness; doses of these should be
spaced
2 or 3 hours apart from doses of scopolamine
methyl nitrate.
•
Anticholinergics – Concurrent use with anticholinergic effects;
patients should be advised to report
occurrence
of gastrointestinal problems promptly since paralytic
ileus may occur with concurrent therapy.
• CNS
Depressants – Concurrent use of alcohol, antihistamines
with alcohol, tricyclic antidepressants,
barbiturates
and other CNS depressants may have an additive
effect.
•
Ketoconazole – Anticholinergics may increase gastrointestinal
pH, possibly resulting in a marked
reduction
in ketoconazole absorption during concurrent
use with anticholinergics; patients should be
advised
to take these medications at least 2 hours after
ketoconazole.
• MAO
inhibitors – Concurrent use may prolong and intensify
cardiac stimulate and vasopressor effects of
chlorpheniramine,
resulting in headache, cardiac arrhythmias,
vomiting or sudden and severe
hypertensive
and/or hyperpyretic crisis. These medications
should not be administered during
or
within 14 days following the administration of MAO inhibitor
therapy.
•
Metoclopramide – Concurrent use of metoclopramide with
anticholinergics may antagonize
metoclopramide’s
effects on gastrointestinal motility.
•
Potassium chloride – Concurrent use with anticholinergics
may increase the severity of
potassium
chloride-induced gastrointestinal lesions.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No data is available on the long-term potential of the components of this product for Carcinogenesis, Mutagenesis or Impairment of Fertility in animals or humans.
Pregnancy:
Category
C: It is not known whether Ryneze®
Liquid can cause fetal harm when administered
to a
pregnant woman or can affect reproduction capacity.
Ryneze® Liquid should be given to a pregnant
woman
only if clearly needed.
Nursing Mothers:
It is
not known whether this drug is excreted
in human milk. Because many drugs are excreted
in
human milk, caution should be exercised when Ryneze Liquid
is administered to a nursing woman.
Pediatric Use:
Safety and effectiveness of Ryneze® Liquid in children below the age of 6 have not been established.
Use
is not recommended for children under six years of age.
A paradoxical reaction characterized by
hyperexcitability
may occur in children taking large doses
of anticholinergics.
Geriatric Use:
Geriatric
patients may respond to usual doses
of anticholinergics with excitement, agitation,
drowsiness,
or confusion. Geriatric patients are especially
susceptible to the anticholinergic side effects,
such
as constipation, dryness of the mouth, and urinary retention
(especially in males). If these side effects occur
and
continue or are severe, medication should probably be
discontinued.
Caution
is also recommended when anticholinergics are given
to geriatric patients, because of the danger of
precipitating
undiagnosed glaucoma. Memory may become
severely impaired in geriatric patients, with the
continued
use of anticholinergics, since these drugs block
the action of acetylcholine, which is responsible for
many
functions of the brain, including memory function.
ADVERSE REACTIONS:
The
following adverse reactions have
been observed with the use of chlorpheniramine and
scopolamine
methyl nitrate; Arrhytmias, blood dyscrasias, CNS
depression, CNS stimulation, dizziness, drowsiness,
dryness
of mouth, hallucinations, hypotension, hypertension,
increased sweating, loss of appetite,
paradoxical
reaction, restlessness, skin rash, stomach upset
or pain, thickening of mucus, tingling in hands or
feet,
trembling, troubled breathing, unusual tiredness or weakness,
vomiting.
Note:
Agitation; confusion; difficult or painful urination; drowsiness;
dizziness; and dryness of mouth, nose and
throat
are more likely to occur in the elderly. Nightmares,
unusual excitement, nervousness,
restlessness,
or irritability are more likely to occur in children
and the elderly. When anticholinergics are given
to
patients, especially children, where the environmental temperature
is high, there is risk of a rapid increase in
body
temperature.
OVERDOSAGE:
In
all cases of suspected overdose, immediately
call your regional poison center and/or
contact
a physician immediately. The stomach should be emptied
promptly by lavage or induction of emesis with
syrup
of ipecac. The installation of activated charcoal into he
stomach also should be considered. The treatment of
overdose
is essentially sypmptomatic and supportive. If respiratory
depression is present treat promptly with oxygen
and/or
mechanical support of ventilation. If convulsions
or marked CNS excitement occurs, only short-acting
benzodiazepine-type
drugs should be used.
DOSAGE AND ADMINISTRATION:
Adults and children 12 years of age and older:
1 teaspoonful (5 mL) every 4 to 6 hours, not to exceed 6 teaspoonfuls in 24 hours.
Children
6 to under 12 years of age:
½ teaspoonful (2.5 mL) every 4 to 6 hours, not to exceed 3 teaspoonfuls in 24 hours.
RYNEZE® LIQUID is not recommended for children under 6 years of age.
Note:Geriatric patients may be more sensitive to the effects of the usual adult dose. Adjust adult dose accordingly.
HOW SUPPLIED:
RYNEZE®
LIQUID is supplied as a clear,
grape-flavored liquid, dye free, sugar free, alcohol
free,
and gluten free in 16 fl oz (473 mL) bottles, NDC
24839-346-16, and 10 mL sample,
NDC
24839-346-10
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF CHILDREN. IN CASE OF ACCIDENTAL
OVERDOSE, CALL A DOCTOR OR CONTACT A POISON CONTROL CENTER IMMEDIATELY.
Pharmacist: Store at controlled room temperature, 15°-30°C
(59°-86°F). Avoid exposure to heat. Dispense
in a
tight, light-resistant container as defined in
the USP/NF with a child-resistant closure.
PRODUCT PACKAGING:
The packaging below represents the labeling currently used.
Ryneze ® Liquid
Antihistamine/Anticholinergic
Each
5 mL (one teaspoonful) for oral
administration
contains:
Chlorpheniramine
Maleate ...... 4 mg
Scopolamine
Methyl Nitrate .... 1.25 mg
Rx Only
Dye Free/Sugar Free
Alcohol Free/Gluten Free
Grape Flavor
16 fl oz (473 mL)
®
:
oooo







Ryneze LiquidChlorpheniramine maleate, Scopolamine Methyl Nitrate LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||