REPAIRWEAR UPLIFTING FIRMING
REPAIRWEAR UPLIFTING FIRMING BROAD SPECTUM SPF 15 - NORMAL COMBO TO COMBINATION OILY
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients
- Purpose
- Use
- Warnings
- Directions
- Inactive ingredients
- REPAIRWEAR UPLIFTING FIRMING Other information
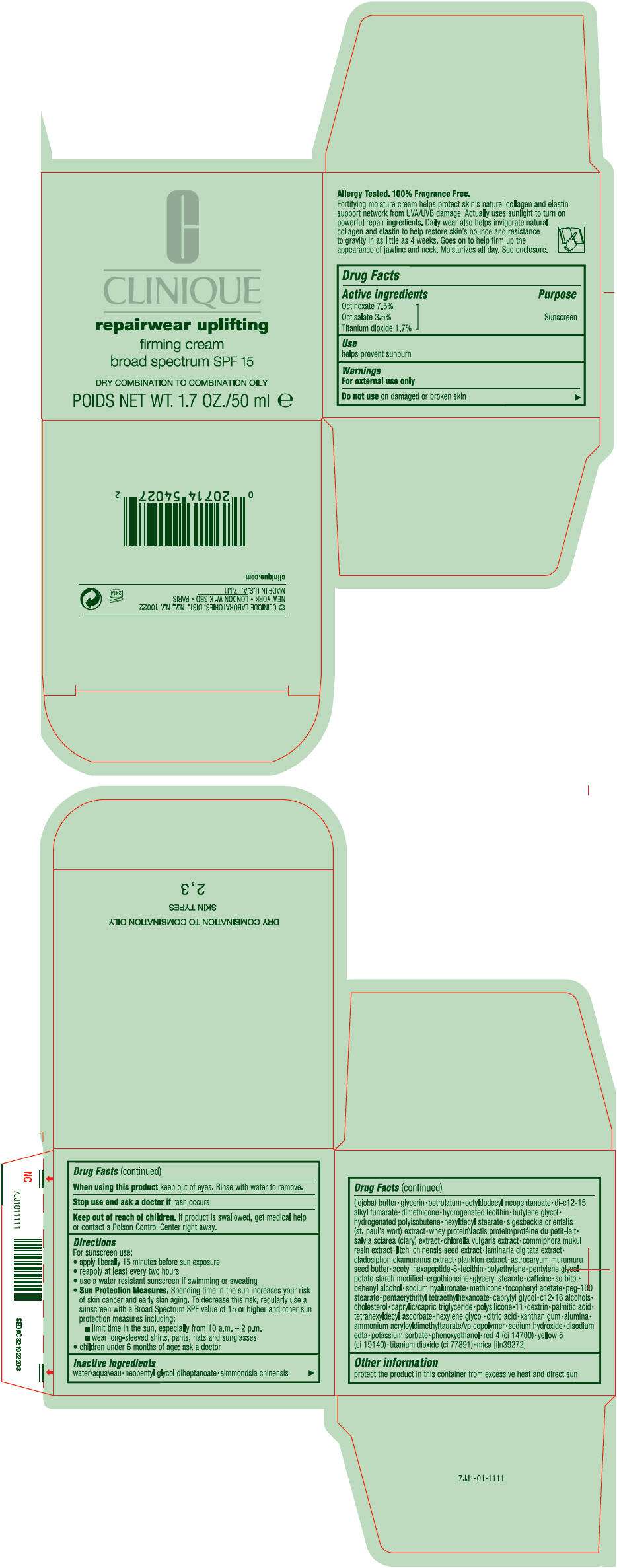
- PRINCIPAL DISPLAY PANEL - 50 mL Jar Carton
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredients
Octinoxate 7.5%
Octisalate 3.5%
Titanium dioxide 1.7%
Purpose
Sunscreen
Use
helps prevent sunburn
Warnings
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.
Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
Inactive ingredients
water\aqua\eau • neopentyl glycol diheptanoate • simmondsia chinensis (jojoba) butter • glycerin • petrolatum • octyldodecyl neopentanoate • di-c12-15 alkyl fumarate • dimethicone • hydrogenated lecithin • butylene glycol • hydrogenated polyisobutene • hexyldecyl stearate • sigesbeckia orientalis (st. paul's wort) extract • whey protein\lactis protein\protéine du petit-lait • salvia sclarea (clary) extract • chlorella vulgaris extract • commiphora mukul resin extract • litchi chinensis seed extract • laminaria digitata extract • cladosiphon okamuranus extract • plankton extract • astrocaryum murumuru seed butter • acetyl hexapeptide-8 • lecithin • polyethylene • pentylene glycol • potato starch modified • ergothioneine • glyceryl stearate • caffeine • sorbitol • behenyl alcohol • sodium hyaluronate • methicone • tocopheryl acetate • peg-100 stearate • pentaerythrityl tetraethylhexanoate • caprylyl glycol • c12-16 alcohols • cholesterol • caprylic/capric triglyceride • polysilicone-11 • dextrin • palmitic acid • tetrahexyldecyl ascorbate • hexylene glycol • citric acid • xanthan gum • alumina • ammonium acryloyldimethyltaurate/vp copolymer • sodium hydroxide • disodium edta • potassium sorbate • phenoxyethanol • red 4 (ci 14700) • yellow 5 (ci 19140) • titanium dioxide (ci 77891) • mica [iln39272]
REPAIRWEAR UPLIFTING FIRMING Other information
protect the product in this container from excessive heat and direct sun
DIST. N.Y., N.Y. 10022
PRINCIPAL DISPLAY PANEL - 50 mL Jar Carton
C
CLINIQUE
repairwear uplifting
firming cream
broad spectrum SPF 15
DRY COMBINATION TO COMBINATION OILY
POIDS NET WT. 1.7. OZ./50 ml e