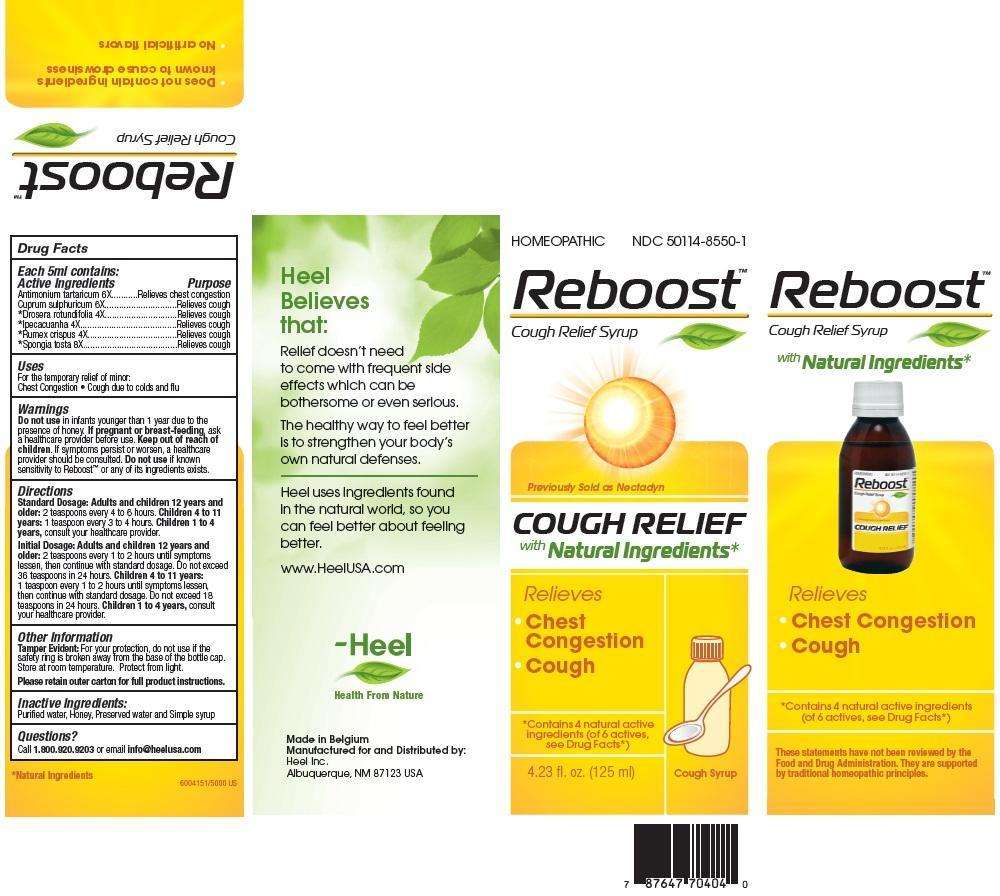
Reboost
Reboost Cough Syrup
FULL PRESCRIBING INFORMATION: CONTENTS*
- ACTIVE INGREDIENTS
- INACTIVE INGREDIENTS
- PURPOSE
- REBOOST DOSAGE AND ADMINISTRATION
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- REBOOST INDICATIONS AND USAGE
FULL PRESCRIBING INFORMATION
ACTIVE INGREDIENTS
Active Ingredients: Each 5ml contains: Antimonium tartaricum 6X, Cuprum sulphuricum 6X, Drosera rotundifolia 4X, Ipecacuanha 4X, Rumex crispus 4X, Spongia tosta 8X
INACTIVE INGREDIENTS
Inactive Ingredients: Purified Water, Honey, Preserved water and Simple syrup
PURPOSE
Antimonium tartaricum 6X..............Relieves chest congestion
Cuprum sulphuricum 6X.................Relieves cough
Drosera rotundifolia 4X..................Relieves cough
Ipecacuanha 4X.............................Relieves cough
Rumex crispus 4X...........................Relieves cough
Spongia tosta 8X...........................Relieves cough
REBOOST DOSAGE AND ADMINISTRATION
Standard Dosage: Adults and children 12 years and older: 2 teaspoons every 4 to 6 hours.
Children 4 to 11 tears: 1 teaspoon every 3 to 4 hours.
Children 1 to 4 years, consult your healthcare provider.
Initial Dosage: Adults and children 12 years and older: 2 teaspoons every 1 to 2 hours until symptoms lessen, then continue with standard dosage. Do not exceed 36 teaspoons in 24 hours.
Children 4 to 11 tears: 1 teaspoon every 1 to 2 hours until symptoms lessen, then continue with standard dosage. Do not exceed 18 teaspoons in 24 hours.
Children 1 to 4 years, consult your healthcare provider.
WARNINGS
Do not use in infants younger than 1 year due to the presence of honey. If pregnant or breast-feeding, ask a healthcare provider before use. Keep out of reach of children. If symptoms persist or worsen, a healthcare provider should be consulted. Do not use if known sensitivity to Reboost or any of its ingredients exists.
KEEP OUT OF REACH OF CHILDREN
Keep out of reach of children.
REBOOST INDICATIONS AND USAGE
For the temporary relief of minor:
- Chest Congestion
- Cough due to colds and flu
ReboostANTIMONY POTASSIUM TARTRATE SYRUP
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||