R-Tanna
PD-Rx Pharmaceuticals, Inc.
PD-Rx Pharmaceuticals, Inc.
R-Tanna Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- Description
- Clinical Pharmacology
- R-Tanna Indications and Usage
- Contraindications
- Warnings
- Precautions
- Side Effects
- Overdosage
- Dosage and Administration
- How Supplied
- Package Label - Principal Display Panel - 100 Tablet Bottle, R-Tanna Tablets
FULL PRESCRIBING INFORMATION
Description
antihistamine/nasal decongestant combination. Each tablet contains:Phenylephrine Tannate 25 mg
Chlorpheniramine Tannate 9 mg
Other ingredients: corn starch, dibasic calcium phosphate, magnesium stearate, methylcellulose, polygalacturonic acid, povidone, talc.
Clinical Pharmacology
R-Tanna Indications and Usage
Contraindications
Warnings
Precautions
General: Antihistamines are more likely to cause dizziness, sedation and hypotension in elderly patients. Antihistamines may cause excitation, particularly in children, but their combination with sympathomimetics may cause either mild stimulation or mild sedation.
Information for patients: Caution patients against drinking alcoholic beverages or engaging in potentially hazardous activities requiring alertness, such as driving a car or operating machinery while using this product. Patients should be warned not to use this product if they are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If patients are uncertain whether a prescription drug contains an MAOI, they should be instructed to consult a health professional before taking such a product.
Drug interactions: MAO inhibitors may prolong and intensify the anticholinergic effects of antihistamines and the overall effects of sympathomimetic agents.
Carcinogenesis, mutagenesis, impairment of fertility: No long term animal studies have been performed with R-Tanna Tablets.
Pregnancy: Teratogenic effects: Pregnancy Category C. Animal reproduction studies have not been conducted with R-Tanna Tablets. It is also not known whether R-Tanna Tablets can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. R-Tanna Tablets should be given to a pregnant woman only if clearly needed.
Nursing mothers: R-Tanna Tablets should not be administered to a nursing woman.
Side Effects
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-800-526-3840 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .Overdosage
Signs and symptoms : May vary from CNS depression to stimulation (restlessness to convulsions). Antihistamine overdosage in young children may lead to convulsions and death. Atropine-like signs and symptoms may be prominent.
Treatment: Induce vomiting if it has not occurred spontaneously. Precautions must be taken against aspiration especially in infants, children and comatose patients. If gastric lavage is indicated, isotonic or half-isotonic saline solution is preferred. Stimulants should not be used. If hypotension is a problem, vasopressor agents may be considered.
Dosage and Administration
How Supplied
Storage:°°°°
To report SUSPECTED ADVERSE REACTIONS, contact Meda Pharmaceuticals Inc. at 1-800-526-3840 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Mfd. For: Prasco Laboratories
Mason, OH 45040 USA
Mfd. By: Meda Pharmaceuticals Inc.
Somerset, New Jersey 08873-4120
Rev. 05/10
Package Label - Principal Display Panel - 100 Tablet Bottle, R-Tanna Tablets
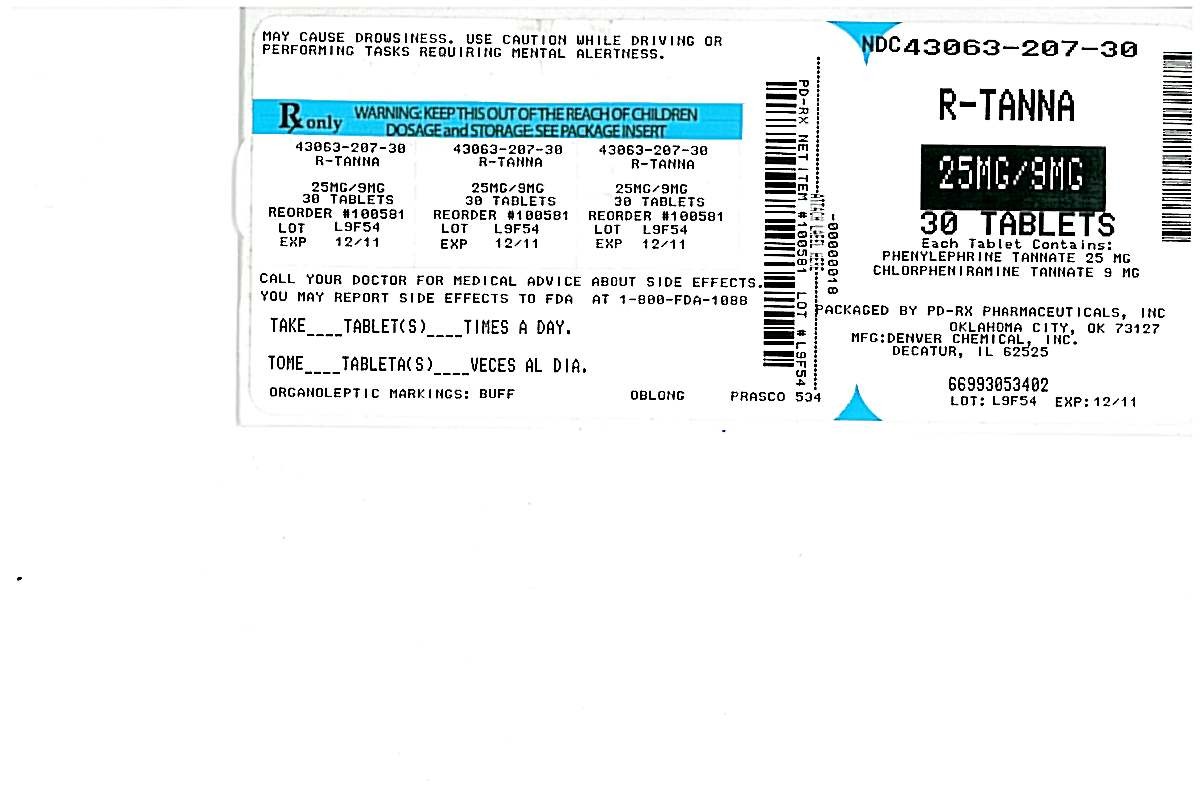
PDRx Label
R-TannaPhenylephrine Tannate and Chlorpheniramine Tannate TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||