Quinapril
Bryant Ranch Prepack
Bryant Ranch Prepack
QUINAPRIL TABLETS, USP, 5 mg, 10 mg, 20 mg, and 40 mg 1050 1051 1045 1053 Rx only
FULL PRESCRIBING INFORMATION: CONTENTS*
- USE IN PREGNANCY
- QUINAPRIL DESCRIPTION
- CLINICAL PHARMACOLOGY
- QUINAPRIL INDICATIONS AND USAGE
- QUINAPRIL CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- QUINAPRIL ADVERSE REACTIONS
- OVERDOSAGE
- QUINAPRIL DOSAGE AND ADMINISTRATION
- Quinapril Hcl 20mg Tablet
FULL PRESCRIBING INFORMATION
USE IN PREGNANCY
When pregnancy is detected, quinapril tablets should be discontinued as soon as possible. See , . When used in pregnancy during the second and third trimesters, ACE inhibitors can cause injury and even death to the developing fetus. WARNINGS Fetal/Neonatal Morbidity and Mortality
QUINAPRIL DESCRIPTION
Quinapril hydrochloride is the hydrochloride salt of quinapril, the ethyl ester of a non-sulfhydryl, angiotensin-converting enzyme (ACE) inhibitor, quinaprilat.
Quinapril hydrochloride is chemically described as [3 -[2[ *( *)],3 *]]-2-[2-[[1-(ethoxycarbonyl)-3-phenylpropyl]amino]-1-oxopropyl]-1,2,3,4-tetrahydro-3-isoquinolinecarboxylic acid, monohydrochloride. Its structural formula is: S R R R
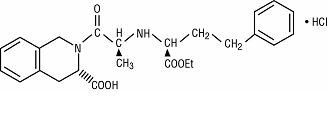
C H N O •HCl M.W. 474.98 25 30 2 5
Quinapril hydrochloride is a white to off-white amorphous powder that is freely soluble in aqueous solvents.
Quinapril tablets, USP contain 5 mg, 10 mg, 20 mg, or 40 mg of quinapril for oral administration. In addition, each tablet also contains the following inactive ingredients: crospovidone, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, FD&C Yellow No. 6 Aluminum Lake, gelatin, hypromellose, lactose monohydrate, magnesium carbonate, magnesium stearate, polyethylene glycol, titanium dioxide, and triacetin.
CLINICAL PHARMACOLOGY
Mechanism of Action
Quinapril is deesterified to the principal metabolite, quinaprilat, which is an inhibitor of ACE activity in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor, angiotensin II. The effect of quinapril in hypertension appears to result primarily from the inhibition of circulating and tissue ACE activity, thereby reducing angiotensin II formation. Quinapril inhibits the elevation in blood pressure caused by intravenously administered angiotensin I, but has no effect on the pressor response to angiotensin II, norepinephrine or epinephrine. Angiotensin II also stimulates the secretion of aldosterone from the adrenal cortex, thereby facilitating renal sodium and fluid reabsorption. Reduced aldosterone secretion by quinapril may result in a small increase in serum potassium. In controlled hypertension trials, treatment with quinapril alone resulted in mean increases in potassium of 0.07 mmol/L (see ). Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity (PRA). PRECAUTIONS
While the principal mechanism of antihypertensive effect is thought to be through the renin-angiotensin-aldosterone system, quinapril exerts antihypertensive actions even in patients with low renin hypertension. Quinapril was an effective antihypertensive in all races studied, although it was somewhat less effective in blacks (usually a predominantly low renin group) than in nonblacks. ACE is identical to kininase II, an enzyme that degrades bradykinin, a potent peptide vasodilator; whether increased levels of bradykinin play a role in the therapeutic effect of quinapril remains to be elucidated.
Pharmacokinetics and Metabolism
Following oral administration, peak plasma quinapril concentrations are observed within one hour. Based on recovery of quinapril and its metabolites in urine, the extent of absorption is at least 60%. The rate and extent of quinapril absorption are diminished moderately (approximately 25 to 30%) when quinapril tablets are administered during a high-fat meal. Following absorption, quinapril is deesterified to its major active metabolite, quinaprilat (about 38% of oral dose), and to other minor inactive metabolites. Following multiple oral dosing of quinapril, there is an effective accumulation half-life of quinaprilat of approximately 3 hours, and peak plasma quinaprilat concentrations are observed approximately 2 hours post-dose. Quinaprilat is eliminated primarily by renal excretion, up to 96% of an IV dose, and has an elimination half-life in plasma of approximately 2 hours and a prolonged terminal phase with a half-life of 25 hours. The pharmacokinetics of quinapril and quinaprilat are linear over a single-dose range of 5 to 80 mg doses and 40 to 160 mg in multiple daily doses. Approximately 97% of either quinapril or quinaprilat circulating in plasma is bound to proteins.
In patients with renal insufficiency, the elimination half-life of quinaprilat increases as creatinine clearance decreases. There is a linear correlation between plasma quinaprilat clearance and creatinine clearance. In patients with end-stage renal disease, chronic hemodialysis or continuous ambulatory peritoneal dialysis has little effect on the elimination of quinapril and quinaprilat. Elimination of quinaprilat may be reduced in elderly patients (≥ 65 years) and in those with heart failure; this reduction is attributable to decrease in renal function (see ). Quinaprilat concentrations are reduced in patients with alcoholic cirrhosis due to impaired deesterification of quinapril. Studies in rats indicate that quinapril and its metabolites do not cross the blood-brain barrier. DOSAGE AND ADMINISTRATION
Pharmacodynamics and Clinical Effects
QUINAPRIL INDICATIONS AND USAGE
QUINAPRIL CONTRAINDICATIONS
Quinapril tablets are contraindicated in patients who are hypersensitive to this product and in patients with a history of angioedema related to previous treatment with an ACE inhibitor.
WARNINGS
PRECAUTIONS
General
Information for Patients
Drug Interactions
Carcinogenesis, Mutagenesis, Impairment of Fertility
Quinapril hydrochloride was not carcinogenic in mice or rats when given in doses up to 75 or 100 mg/kg/day (50 to 60 times the maximum human daily dose, respectively, on an mg/kg basis and 3.8 to 10 times the maximum human daily dose when based on an mg/m basis) for 104 weeks. Female rats given the highest dose level had an increased incidence of mesenteric lymph node hemangiomas and skin/subcutaneous lipomas. Neither quinapril nor quinaprilat were mutagenic in the Ames bacterial assay with or without metabolic activation. Quinapril was also negative in the following genetic toxicology studies: mammalian cell point mutation, sister chromatid exchange in cultured mammalian cells, micronucleus test with mice, chromosome aberration with V79 cultured lung cells, and in an cytogenetic study with rat bone marrow. There were no adverse effects on fertility or reproduction in rats at doses up to 100 mg/kg/day (60 and 10 times the maximum daily human dose when based on mg/kg and mg/m , respectively). 2 in vitro in vitro in vivo 2
Pregnancy
Teratogenic Effects
Nursing Mothers
Because quinapril is secreted in human milk, caution should be exercised when this drug is administered to a nursing woman.
Pediatric Use
The safety and effectiveness of quinapril in pediatric patients have not been established.
Geriatric Use
Clinical studies of quinapril did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly patients exhibited increased area under the plasma concentration time curve and peak levels for quinaprilat compared to values observed in younger patients; this appeared to relate to decreased renal function rather than to age itself.
QUINAPRIL ADVERSE REACTIONS
Clinical Laboratory Test Findings
OVERDOSAGE
Doses of 1440 to 4280 mg/kg of quinapril cause significant lethality in mice and rats.
No specific information is available on the treatment of overdosage with quinapril. The most likely clinical manifestation would be symptoms attributable to severe hypotension.
Laboratory determinations of serum levels of quinapril and its metabolites are not widely available, and such determinations have, in any event, no established role in the management of quinapril overdose.
No data are available to suggest physiological maneuvers (e.g., maneuvers to change pH of the urine) that might accelerate elimination of quinapril and its metabolites.
Hemodialysis and peritoneal dialysis have little effect on the elimination of quinapril and quinaprilat. Angiotensin II could presumably serve as a specific antagonist-antidote in the setting of quinapril overdose, but angiotensin II is essentially unavailable outside of scattered research facilities. Because the hypotensive effect of quinapril is achieved through vasodilation and effective hypovolemia, it is reasonable to treat quinapril overdose by infusion of normal saline solution.
QUINAPRIL DOSAGE AND ADMINISTRATION
Quinapril Hcl 20mg Tablet

QuinaprilQuinapril TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||