QNASL
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use QNASL Nasal Aerosol safely and effectively. See full prescribing information for QNASL Nasal Aerosol. QNASL™ (beclomethasone dipropionate) Nasal Aerosol For Intranasal Use Only Initial U.S. Approval: 1976INDICATIONS AND USAGEQNASL Nasal Aerosol is indicated for the treatment of nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and adolescents 12 years of age and older. ( 1.1 )DOSAGE AND ADMINISTRATIONQNASL Nasal Aerosol is for intranasal use only. The recommended dose of QNASL Nasal Aerosol is 320 mcg per day administered as 2 nasal aerosol sprays in each nostril once daily (maximum total daily dose of 4 nasal aerosol sprays per day). ( 2.1 ) DOSAGE FORMS AND STRENGTHSNasal Aerosol: Each actuation delivers 80 mcg of beclomethasone dipropionate. ( 3 ) Supplied in an 8.7 g canister containing 120 actuations. ( 16 ) CONTRAINDICATIONSPatients with a history of hypersensitivity to beclomethasone dipropionate and/or any other QNASL Nasal Aerosol ingredients. ( 4 )WARNINGS AND PRECAUTIONS Nasal discomfort, epistaxis, nasal ulceration, Candida albicans infection, nasal septal perforation, impaired wound healing. Monitor patients periodically for signs of adverse effects on the nasal mucosa. Avoid use in patients with recent nasal ulcers, nasal surgery, or nasal trauma. ( 5.1 ) Development of glaucoma or posterior subcapsular cataracts. Monitor patients closely with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts. ( 5.2 ) Hypersensitivity, rash, and urticaria may occur after administration of QNASL Nasal Aerosol. ( 5.3 ) Potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex. More serious or even fatal course of chickenpox or measles in susceptible patients. Use caution in patients with the above because of the potential for worsening of these infections. ( 5.4 ) Hypercorticism and adrenal suppression with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue QNASL Nasal Aerosol slowly. ( 5.5 ) Potential reduction in growth velocity in pediatric patients. Monitor growth routinely in pediatric patients receiving QNASL Nasal Aerosol. ( 5.6, 8.4 ) Side EffectsThe most common adverse reactions (≥ 1% and greater than placebo) include nasal discomfort, epistaxis, and headache. ( 6.1 ) To report SUSPECTED ADVERSE REACTIONS, contact Teva Respiratory, LLC at 1-888-482-9522 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 QNASL INDICATIONS AND USAGE
- 2 QNASL DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 QNASL CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 QNASL ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 10 OVERDOSAGE
- 11 QNASL DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- AND INSTRUCTIONS FOR USE LEAFLET
- PRINCIPal DISPLAY PANEL, Part 1 of 2
- PRINCIPal DISPLAY PANEL, Part 2 of 2
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Treatment of Nasal Symptoms of Allergic Rhinitis
QNASL Nasal Aerosol is indicated for the treatment of the nasal symptoms associated with seasonal and perennial allergic rhinitis in adults and adolescents 12 years of age and older.
2 DOSAGE AND ADMINISTRATION
Administer QNASL Nasal Aerosol by the intranasal route only. QNASL Nasal Aerosol must be primed prior to initial use by actuating four times. To do this, remove the protective dust cap from the device, hold the device upright between your thumb and forefinger (index finger) (the canister should be on top, pointing down), and spray 4 times into the air, away from your eyes and face. After the initial priming, the dose counter should read 120. If QNASL Nasal Aerosol is not used for 7 consecutive days it should be primed by spraying 2 times. See accompanying illustrated Patient Information and Instructions for Use leaflet for proper use of QNASL Nasal Aerosol.
2.1 Allergic Rhinitis
Adults and Adolescents (12 Years of Age and Older): The recommended dose of QNASL Nasal Aerosol is 320 mcg per day administered as 2 nasal aerosol sprays in each nostril (80 mcg/aerosol spray) once daily (maximum total daily dose of 4 nasal aerosol sprays per day).
3 DOSAGE FORMS AND STRENGTHS
QNASL Nasal Aerosol is a nonaqueous nasal spray solution. Each actuation of the aerosol delivers 80 mcg of beclomethasone dipropionate.
4 CONTRAINDICATIONS
QNASL Nasal Aerosol is contraindicated in patients with a history of hypersensitivity to beclomethasone dipropionate and/or any other QNASL Nasal Aerosol ingredients [see Warnings and Precautions (5.3) ].
5 WARNINGS AND PRECAUTIONS
5.1 Local Nasal Effects
Nasal Discomfort, Epistaxis, and Nasal Ulceration: In clinical trials of 2 to 52 weeks duration, epistaxis and nasal ulcerations were observed more frequently and some epistaxis events were more severe in patients treated with QNASL Nasal Aerosol than those who received placebo. In the 52-week safety trial in patients with perennial allergic rhinitis, nasal erosions were identified in 4 of 415 patients and a nasal ulceration was identified in 1 of 415 patients treated with QNASL Nasal Aerosol. No nasal erosions or ulcerations were reported for patients who received placebo. Patients using QNASL Nasal Aerosol over several months or longer should be examined periodically for possible changes in the nasal mucosa. If an adverse reaction (e.g., erosion, ulceration) is noted, discontinue QNASL Nasal Aerosol. [see Adverse Reactions (6.1) ].
Candida Infection: In previous clinical trials with an aqueous formulation of beclomethasone dipropionate administered intranasally, localized infections of the nose and pharynx with Candida albicans had been reported. There were no instances of similar infections observed in clinical trials with QNASL Nasal Aerosol. If such an infection develops, it may require treatment with appropriate local therapy and discontinuation of QNASL Nasal Aerosol treatment. Thus, patients using QNASL Nasal Aerosol over several months or longer should be examined periodically for evidence of Candida infection.
Nasal Septal Perforation: Instances of nasal septal perforation have been reported in patients following the intranasal application of beclomethasone dipropionate. There were no instances of nasal septal perforation observed in clinical trials with QNASL Nasal Aerosol.
Impaired Wound Healing: Because of the inhibitory effect of corticosteroids on wound healing, patients who have experienced recent nasal septal ulcers, nasal surgery, or nasal trauma should not use QNASL Nasal Aerosol until healing has occurred.
5.2 Glaucoma and Cataracts
Use of intranasal and inhaled corticosteroids may result in the development of glaucoma and/or cataracts. Therefore, close monitoring is warranted in patients with a change in vision or with a history of increased intraocular pressure, glaucoma, and/or cataracts.
Glaucoma and cataract formation was evaluated with ocular assessments that included intraocular pressure measurements and slit lamp examinations in 245 adolescent and adult patients (12 years of age and older) with perennial allergic rhinitis who were treated with QNASL Nasal Aerosol 320 mcg daily (N=197) or placebo (N=48) for up to 52 weeks. In 94% of patients, intraocular pressure (IOP) remained within the normal range (<21 mmHg) during the treatment portion of the trial. There were 10 patients (5%) treated with QNASL Nasal Aerosol and 1 patient (2%) treated with placebo that had intraocular pressure that increased above normal levels (≥21 mmHg) and greater than baseline during the treatment portion of the trial. Two of these occurrences in patients treated with QNASL Nasal Aerosol were reported as adverse reactions, one serious. No instances of cataract formation or other clinically significant ocular incidents were reported in this 52-week safety trial.[see Adverse Reactions (6.1) ].
5.3 Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis, angioedema, urticaria, and rash have been reported following administration of beclomethasone dipropionate nasally administered and inhalationally administered products. Angioedema, urticaria, and rash have been reported following administration of QNASL Nasal Aerosol. Discontinue QNASL Nasal Aerosol if any such reactions occur [see Contraindications (4) ].
5.4 Immunosuppression
Persons who are using drugs that suppress the immune system (e.g., corticosteroids) are more susceptible to infections than healthy individuals. Chickenpox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affect the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If a patient is exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see the respective package inserts for complete VZIG and IG prescribing information). If chickenpox or measles develops, treatment with antiviral agents may be considered.
Corticosteroids should be used with caution, if at all, in patients with active or quiescent tuberculous infections of the respiratory tract, untreated local or systemic fungal or bacterial infections, systemic viral or parasitic infections, or ocular herpes simplex because of the potential for worsening of these infections.
5.5 Hypothalamic-Pituitary-Adrenal Axis Effect
When intranasal steroids are used at higher-than-recommended dosages or in susceptible individuals at recommended dosages, systemic corticosteroid effects such as hypercorticism and adrenal suppression may appear. If such changes occur, the dosage of QNASL Nasal Aerosol should be discontinued slowly, consistent with accepted procedures for discontinuing oral corticosteroid therapy.
The replacement of a systemic corticosteroid with a topical corticosteroid can be accompanied by signs of adrenal insufficiency. In addition, some patients may experience symptoms of corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, and depression). Patients previously treated for prolonged periods with systemic corticosteroids and transferred to topical corticosteroids should be carefully monitored for acute adrenal insufficiency in response to stress. In patients who have asthma or other clinical conditions requiring long-term systemic corticosteroid treatment, rapid decreases in systemic corticosteroid dosages may cause a severe exacerbation of their symptoms.
5.6 Effect on Growth
Corticosteroids may cause a reduction in growth velocity when administered to pediatric patients. Routinely monitor the growth of pediatric patients receiving QNASL Nasal Aerosol [see Use in Specific Populations (8.4) ].
6 ADVERSE REACTIONS
Systemic and local corticosteroid use may result in the following:
- Epistaxis, nasal discomfort, nasal ulcerations, Candida albicans infection, and impaired wound healing [see Warnings and Precautions (5.1) ]
- Glaucoma and cataracts [see Warnings and Precautions (5.2) ]
- Hypercorticism, adrenal suppression, and growth reduction [see Warnings and Precautions (5.5) (5.6) , Use in Specific Populations (8.4) ]
- Immunosuppression [see Warnings and Precautions (5.4) ]
6.1 Clinical Trials Experience
The safety data described below for adults and adolescents 12 years of age and older with seasonal or perennial allergic rhinitis are based on 4 placebo-controlled clinical trials of 2 to 6 weeks duration evaluating doses of beclomethasone nasal aerosol from 80 to 320 mcg once daily. These short-term trials included a total of 1394 patients with either seasonal or perennial allergic rhinitis. Of these, 575 (378 female and 197 male) received at least one dose of QNASL Nasal Aerosol, 320 mcg once daily and 578 (360 female and 218 male) received placebo. Patient ages ranged from 12 to 82 years and the racial distribution of patients was 81% white, 16% black, and 4% other.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Short-Term (2–6 Weeks) Trials: Overall, the incidence of adverse reactions did not differ appreciably between patients treated with QNASL Nasal Aerosol and those who were treated with placebo. Less than 2% of patients in the clinical trials discontinued treatment because of adverse reactions with the rate of withdrawal among patients who received QNASL Nasal Aerosol similar to or lower than the rate among patients who received placebo. Table 1 displays the common adverse reactions (≥ 1% and greater than placebo-treated patients).
|
QNASL Nasal Aerosol 320 mcg (N = 575) n (%) |
Placebo (N = 578) n (%) |
|
| Nasal Discomfort | 30 (5.2) | 28 (4.8) |
| Epistaxis | 11 (1.9) | 7 (1.2) |
| Headache | 13 (2.3) | 9 (1.6) |
Nasal ulcerations occurred in 2 patients treated with placebo and in 1 patient treated with QNASL Nasal Aerosol. There were no differences in the incidence of adverse reactions based on gender or race. Clinical trials did not have sufficient numbers of patients aged 65 years and older to determine whether they respond differently than younger patients.
Long-Term 52-Week Safety Trial: In a 52-week placebo-controlled long-term safety trial in patients with PAR, 415 patients (128 males and 287 females, aged 12 to 74 years) were treated with QNASL Nasal Aerosol at a dose of 320 mcg once daily and 111 patients (44 males and 67 females, aged 12 to 67 years) were treated with placebo. Of the 415 patients treated with QNASL Nasal Aerosol, 219 patients were treated for 52 weeks and 196 patients were treated for 30 weeks. While most adverse events were similar in type and rate between the treatment groups, epistaxis occurred more frequently in patients who received QNASL Nasal Aerosol (45 out of 415, 11%) than in patients who received placebo (2 out of 111, 2%). Epistaxis also tended to be more severe in patients treated with QNASL Nasal Aerosol. In 45 reports of epistaxis in patients who received QNASL Nasal Aerosol, 27, 13, and 5 cases were of mild, moderate, and severe intensity, respectively, while the reports of epistaxis in patients who received placebo were of mild (1) and moderate (1) intensity. Seventeen patients treated with QNASL Nasal Aerosol experienced adverse reactions that led to withdrawal from the trial compared to 3 patients treated with placebo. There were 4 nasal erosions and 1 nasal septum ulceration which occurred in patients who received QNASL Nasal Aerosol, and no erosions or ulcerations noted in patients who received placebo. No patient experienced a nasal septum perforation during the trial.
6.2 Postmarketing Experience
In addition to adverse reactions reported from clinical trials for QNASL Nasal Aerosol, the following adverse events have been reported during use of other intranasal and inhaled formulations of beclomethasone dipropionate. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. These events have been chosen for inclusion due to either their seriousness, frequency of reporting, or causal connection to beclomethasone dipropionate or a combination of these factors.
Intranasal beclomethasone dipropionate: Nasal septal perforation, glaucoma, cataracts, loss of taste and smell, and hypersensitivity reactions including anaphylaxis, angioedema, rash, and urticaria have been reported following intranasal administration of beclomethasone dipropionate.
Inhaled beclomethasone dipropionate: Hypersensitivity reactions, including anaphylaxis, angioedema, rash, urticaria, and bronchospasm have been reported following the oral inhalation of beclomethasone dipropionate.
7 DRUG INTERACTIONS
No drug interaction studies have been performed with QNASL Nasal Aerosol.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects: Pregnancy Category C
There are no adequate and well-controlled clinical trials in pregnant women treated with QNASL Nasal Aerosol. Beclomethasone dipropionate was teratogenic and embryocidal in the mouse and rabbit although these effects were not observed in rats. QNASL Nasal Aerosol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Experience with oral corticosteroids since their introduction in pharmacologic, as opposed to physiologic, doses suggests that rodents are more prone to teratogenic effects from corticosteroids than humans.
Beclomethasone dipropionate administered subcutaneously was teratogenic and embryocidal in the mouse and rabbit at doses approximately twice the maximum recommended human daily intranasal dose (MRHDID) in adults (on a mg/m2 basis at maternal doses of 0.1 and 0.025 mg/kg/day in mice and rabbits, respectively). No teratogenicity or embryocidal effects were seen in rats at approximately 460 times MRHDID (in adults on a mg/m2 basis at a maternal inhalation dose of 15 mg/kg/day).
Non-teratogenic Effects: Hypoadrenalism may occur in infants born of mothers receiving corticosteroids during pregnancy. Such infants should be carefully monitored.
8.3 Nursing Mothers
It is not known whether beclomethasone dipropionate is excreted in human breast milk. However, other corticosteroids have been detected in human breast milk and thus caution should be exercised when QNASL Nasal Aerosol is administered to a nursing mother.
8.4 Pediatric Use
The safety and effectiveness for seasonal and perennial allergic rhinitis in children 12 years of age and older have been established. Controlled clinical trials with QNASL Nasal Aerosol included 188 adolescent patients 12 to 17 years of age [see Clinical Studies (14) ]. The safety and effectiveness of QNASL Nasal Aerosol in children younger than 12 years of age have not been established.
Controlled clinical trials have shown that intranasal corticosteroids may cause a reduction in growth velocity in pediatric patients. This effect has been observed in the absence of laboratory evidence of hypothalamic-pituitary-adrenal (HPA) axis suppression, suggesting that growth velocity is a more sensitive indicator of systemic corticosteroid exposure in pediatric patients than some commonly used tests of HPA-axis function. The long-term effects of reduction in growth velocity associated with intranasal corticosteroids, including the impact on final adult height, are unknown. The potential for "catch-up" growth following discontinuation of treatment with intranasal corticosteroids has not been adequately studied. The growth of pediatric patients receiving intranasal corticosteroids, including QNASL Nasal Aerosol, should be monitored routinely (e.g., via stadiometry).
A 12-month, randomized, controlled clinical trial evaluated the effects of QVAR®, an orally inhaled HFA beclomethasone dipropionate product, without spacer versus chlorofluorocarbon-propelled (CFC) beclomethasone dipropionate with large volume spacer on growth in children with asthma ages 5 to 11 years. A total of 520 patients were enrolled, of whom 394 received HFA-beclomethasone dipropionate (100 to 400 mcg/day ex-valve) and 126 received CFC-beclomethasone dipropionate (200 to 800 mcg/day ex-valve). When comparing results at month 12 to baseline, the mean growth velocity in children treated with HFA-beclomethasone dipropionate was approximately 0.5 cm/year less than that noted with children treated with CFC-beclomethasone dipropionate via large volume spacer. The potential growth effects of prolonged treatment should be weighed against the clinical benefits obtained and the risks/benefits of treatment alternatives.
The potential for QNASL Nasal Aerosol to cause reduction in growth velocity in susceptible patients or when given at higher than recommended dosages cannot be ruled out.
8.5 Geriatric Use
Clinical trials of QNASL Nasal Aerosol did not include sufficient numbers of subjects aged 65 years and older to determine whether they responded differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, administration to elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10 OVERDOSAGE
Chronic overdosage may result in signs/symptoms of hypercorticism [see Warnings and Precautions (5.5) ]. There are no data available on the effects of acute or chronic overdosage with QNASL Nasal Aerosol.
11 DESCRIPTION
Beclomethasone dipropionate USP, the active component of QNASL Nasal Aerosol, is an anti-inflammatory steroid having the chemical name 9-chloro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17, 21-dipropionate and the following chemical structure:
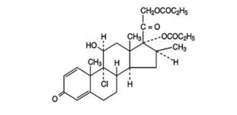
Beclomethasone dipropionate, a di-ester of beclomethasone (a synthetic corticosteroid chemically related to dexamethasone), is a white to almost white, odorless powder with a molecular formula of C28H37ClO7 and a molecular weight of 521.1. It is practically insoluble in water, very soluble in chloroform, and soluble in acetone and in dehydrated alcohol.
QNASL Nasal Aerosol is a pressurized, nonaqueous solution in a metered-dose aerosol device intended ONLY for intranasal use. It contains a solution of beclomethasone dipropionate in propellant HFA-134a (1,1,1,2-tetrafluoroethane) and dehydrated ethanol. Each actuation delivers 100 mcg of beclomethasone dipropionate in 59 mg of solution from the valve and delivers 80 mcg of beclomethasone dipropionate from the nasal actuator. Each canister contains 8.7 g of drug and excipients and provides 120 actuations after priming.
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Beclomethasone dipropionate is a prodrug that is extensively converted to the active metabolite, beclomethasone-17-monopropionate. The precise mechanism through which beclomethasone dipropionate affects rhinitis symptoms is unknown. Corticosteroids have been shown to have multiple anti-inflammatory effects, inhibiting both inflammatory cells (e.g., mast cells, eosinophils, basophils, lymphocytes, macrophages, and neutrophils) and the release of inflammatory mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines).
Beclomethasone-17-monopropionate has been shown in vitro to exhibit a binding affinity for the human glucocorticoid receptor which is approximately 13 times that of dexamethasone, 6 times that of triamcinolone acetonide, 1.5 times that of budesonide and 25 times that of beclomethasone dipropionate. The clinical significance of these findings is unknown.
12.2 Pharmacodynamics
Adrenal Function: The effects of QNASL Nasal Aerosol on the HPA axis were evaluated in a 6-week, randomized, double-blind, parallel-group trial in adult and adolescent patients 12 to 45 years of age with perennial allergic rhinitis. QNASL Nasal Aerosol 320 mcg, once daily, was compared with both placebo nasal aerosol and a positive control (a placebo/prednisone group that received prednisone 10 mg orally once daily for the final 7 days of the treatment period). HPA-axis function was assessed by 24-hour serial serum cortisol levels prior to the first dose and after 6 weeks of treatment. Patients were domiciled for the 24-hour serum cortisol assessments. The change from baseline in the 24-hour serum cortisol weighted mean for QNASL Nasal Aerosol and placebo after 6 weeks of treatment were compared. Baseline geometric mean serum cortisol weighted mean values were similar in the QNASL Nasal Aerosol 320 mcg/day and placebo treatment groups (9.04 and 8.45 mcg/dL, respectively). After 6 weeks of treatment, the geometric mean values were 8.18 and 8.01 mcg/dL, respectively, with a change from baseline in 24-hour serum cortisol weighted mean for the QNASL Nasal Aerosol and placebo groups of 0.86 and 0.44, resulting in a difference of 0.42. The geometric mean ratio for QNASL Nasal Aerosol 320 mcg/day to placebo was 0.96 (95% CI: 0.87, 1.06). For comparison, in the positive-control (prednisone) treatment group, the geometric mean ratio for placebo to placebo/prednisone 10 mg/day was 3.17 (95% CI: 2.68, 3.74).
12.3 Pharmacokinetics
Absorption
Following intranasal administration, most of the beclomethasone dipropionate undergoes extensive conversion to its active metabolite, beclomethasone-17-monopropionate, during absorption. Plasma concentrations of beclomethasone dipropionate and beclomethasone-17-monopropionate have been measured in 2 clinical trials with QNASL Nasal Aerosol.
The single-dose pharmacokinetics of QNASL Nasal Aerosol were evaluated in a randomized, open-label, 3-period, crossover trial in healthy volunteers. Systemic levels of beclomethasone-17-monopropionate and beclomethasone dipropionate after single-dose intranasal administration of beclomethasone dipropionate at doses of 80 and 320 mcg were compared with the systemic levels of beclomethasone-17-monopropionate and beclomethasone dipropionate after administration of orally inhaled beclomethasone dipropionate HFA at a dose of 320 mcg (QVAR® Inhalation Aerosol). The results of this trial demonstrated that the systemic bioavailability of QNASL Nasal Aerosol 320 mcg was approximately 27.5% of that of orally inhaled beclomethasone dipropionate HFA 320 mcg/day based on the plasma concentrations of beclomethasone-17-monopropionate (AUClast: 1139.7 vs 4140.3 hr*pg/mL; GMR: 0.275; 90% CI for the GMR: 0.214, 0.354). The peak exposure to QNASL Nasal Aerosol 320 mcg/day was approximately 19.5% of that of orally inhaled beclomethasone dipropionate HFA 320 mcg/day as measured by beclomethasone-17-monopropionate (Cmax: 262.7 vs 1343.7 pg/mL; GMR: 0.195; 90% CI for the GMR: 0.158, 0.241).
Following repeated once-daily administration of QNASL Nasal Aerosol, there was no accumulation or increase in plasma exposure to beclomethasone-17-monopropionate or beclomethasone dipropionate, most likely due to the short plasma half-life relative to the dosing frequency.
Distribution
The in vitro protein binding for beclomethasone-17-monopropionate was reported to be 94% to 96% over the concentration range of 1000 to 5000 pg/mL. The volume of distribution at steady state for beclomethasone dipropionate is moderate (20 L) but more extensive for beclomethasone-17-monopropionate (424 L).
Metabolism
Beclomethasone dipropionate undergoes extensive first-pass metabolism, forming three metabolites via CYP3A4, beclomethasone-17-monopropionate, beclomethasone-21-monopropionate, and beclomethasone. Beclomethasone-17-monopropionate is the major and most active metabolite.
Elimination
The major route of elimination of inhaled beclomethasone dipropionate appears to be via metabolism. More than 90% of inhaled beclomethasone dipropionate is found as beclomethasone-17-monopropionate in the systemic circulation. The mean elimination half-life of beclomethasone-17-monopropionate is 2.8 hours. The terminal elimination half-lives of beclomethasone dipropionate and beclomethasone-17-monopropionate following intranasal dosing with QNASL Nasal Aerosol (320 mcg) were approximately 0.3 hours and 4.5 hours, respectively. Irrespective of the route of administration (injection, oral, or inhalation), beclomethasone dipropionate and its metabolites are mainly excreted in the feces. Less than 10% of the drug and its metabolites are excreted in the urine. It is likely that intranasal beclomethasone dipropionate follows a similar elimination pathway.
Special Populations
Formal pharmacokinetic studies using QNASL Nasal Aerosol were not conducted in any special populations.
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenicity of beclomethasone dipropionate was evaluated in rats that were exposed for a total of 95 weeks: 13 weeks at inhalation doses up to 0.4 mg/kg and the remaining 82 weeks at combined oral and inhalation doses up to 2.4 mg/kg. In this trial, there was no evidence of carcinogenicity at the highest dose: approximately 70 times the maximum recommended human daily intranasal dose (MRHDID) in adults on a mg/m2 basis.
Beclomethasone dipropionate did not induce gene mutation in bacterial cells or mammalian Chinese hamster ovary (CHO) cells in vitro. No significant clastogenic effect was seen in cultured CHO cells in vitro or in the mouse micronucleus test in vivo.
In rats, beclomethasone dipropionate caused decreased conception rates at an oral dose of 16 mg/kg (approximately 490 times the MRHDID in adults on a mg/m2 basis). There was no significant effect of beclomethasone dipropionate on fertility in rats at oral doses of 1.6 mg/kg (approximately 50 times the MRHDID in adults on a mg/m2 basis). Inhibition of the estrous cycle in dogs was observed following oral doses of 0.5 mg/kg (approximately 50 times the MRHDID in adults on a mg/m2 basis). No inhibition of the estrous cycle in dogs was seen following 12 months of exposure at an estimated inhalation dose of 0.33 mg/kg (approximately 35 times the MRHDID in adults on a mg/m2 basis).
14 CLINICAL STUDIES
14.1 Seasonal and Perennial Allergic Rhinitis
Adult and Adolescent Patients Aged 12 Years and Older: The efficacy and safety of QNASL Nasal Aerosol have been evaluated in 3 randomized, double-blind, parallel-group, multicenter, placebo-controlled clinical trials of 2 to 6 weeks duration in adult and adolescent patients 12 years and older with symptoms of seasonal or perennial allergic rhinitis. The 3 clinical trials included one 2-week dose-ranging trial in patients with seasonal allergic rhinitis, one 2-week efficacy trial in patients with seasonal allergic rhinitis, and one 6-week efficacy trial in patients with perennial allergic rhinitis. The trials included a total of 1049 patients (366 males and 683 females). About 81% of patients were Caucasian and 17% African American, the mean age was approximately 38 years. Of these patients 521 received QNASL Nasal Aerosol 320 mcg once daily administered as 2 sprays in each nostril.
Assessment of efficacy was based on the total nasal symptom score (TNSS). TNSS is calculated as the sum of the patients' scoring of the 4 individual nasal symptoms (rhinorrhea, sneezing, nasal congestion, and nasal itching) on a 0 to 3 categorical severity scale (0 = absent, 1 = mild, 2 = moderate, 3 = severe) as reflective (rTNSS) or instantaneous (iTNSS). rTNSS required the patients to record symptom severity over the previous 12 hours; iTNSS required the patients to record symptom severity over the previous 10 minutes. Morning and evening TNSS scores were averaged over the treatment period and the difference from placebo in the change from baseline rTNSS was the primary efficacy endpoint. The morning iTNSS reflects the TNSS at the end of the 24-hour dosing interval and is an indication of whether the effect was maintained over the 24-hour dosing interval.
Dose-Ranging Trial: The dose-ranging trial was a 2-week trial that evaluated the efficacy of 3 doses of beclomethasone dipropionate nasal aerosol (80, 160, and 320 mcg, once daily) in patients with seasonal allergic rhinitis. In this trial, only treatment with beclomethasone dipropionate nasal aerosol at the dose of 320 mcg/day resulted in statistically significant improvements compared with placebo in the primary efficacy endpoint, rTNSS (Table 2).
| Treatment | N |
Baseline (SD) |
LS Mean (SE) Change from Baseline | Difference From Placebo | ||
| LS Mean | 95% CI | p Value | ||||
|
Beclomethasone dipropionate 320 mcg/day |
122 | 9.17 (1.66) | -2.22 (0.18) | -0.63 | -1.13, 0.13 | 0.013 |
|
Beclomethasone dipropionate 160 mcg/day |
123 | 9.24 (1.57) | -1.87 (0.18) | -0.29 | -0.78, 0.21 | 0.257 |
|
Beclomethasone dipropionate 80 mcg/day |
118 | 9.33 (1.72) | -1.88 (0.18) | -0.29 | -0.80, 0.21 | 0.255 |
| Placebo | 123 | 8.98 (1.47) | -1.59 (0.18) | |||
The 320 mcg dose also demonstrated a statistically significant decrease in morning iTNSS than placebo, indicating that the effect was maintained over the 24-hour dosing interval.
Seasonal and Perennial Allergic Rhinitis Trials: In 2 randomized, double-blind, parallel-group, multicenter, placebo-controlled efficacy trials, once-daily treatment with QNASL Nasal Aerosol for 2 weeks in patients with seasonal allergic rhinitis and for 6 weeks in patients with perennial allergic rhinitis resulted in statistically significant greater decreases from baseline in the rTNSS and morning iTNSS than placebo (Table 3).
| Treatment |
N |
Baseline (SD) |
LS Mean (SE) Change from Baseline | Difference From Placebo | ||
| LS Mean | 95% CI | p Value | ||||
| Seasonal Allergic Rhinitis | ||||||
| Reflective Total Nasal Symptom Scores (rTNSS) | ||||||
| Beclomethasone dipropionate 320 mcg/day |
167 | 9.6 (1.51) | -2.0 (0.16) | -0.91 | -1.3, -0.5 | <0.001 |
| Placebo | 171 | 9.5 (1.54) | -1.0 (0.15) | |||
| Instantaneous Total Nasal Symptom Scores (iTNSS) | ||||||
| Beclomethasone dipropionate 320 mcg/day |
167 | 9.0 (1.74) | -1.7 (0.15) | -0.92 | -1.3, -0.5 | <0.001 |
| Placebo | 171 | 8.7 (1.81) | -0.8 (0.15) | |||
| Perennial Allergic Rhinitis | ||||||
| Reflective Total Nasal Symptom Scores (rTNSS) | ||||||
| Beclomethasone dipropionate 320 mcg/day |
232 | 8.9 (1.70) | -2.5 (0.14) | -0.84 | -1.2, -0.5 | <0.001 |
| Placebo | 234 | 9.0 (1.73) | -1.6 (0.14) | |||
| Instantaneous Total Nasal Symptom Scores (iTNSS) | ||||||
| Beclomethasone dipropionate 320 mcg/day |
232 | 8.1 (1.98) | -2.1 (0.13) | -0.78 | -1.1, -0.4 | <0.001 |
| Placebo | 234 | 8.3 (1.96) | -1.4 (0.13) | |||
16 HOW SUPPLIED/STORAGE AND HANDLING
QNASL Nasal Aerosol is supplied as a pressurized aluminum canister inserted into a blue and white plastic nasal actuator with a built-in dose counter and white dust cap. Each canister contains 8.7 g of drug and excipients and provides 120 actuations (NDC 59310-210-12). Each actuation delivers 80 mcg of beclomethasone dipropionate from the nasal actuator and 100 mcg from the valve.
QNASL Nasal Aerosol has a built-in spray counter, which starts at 124 and counts down each time an aerosol spray is released. The correct amount of medication in each intranasal dose cannot be ensured after the counter reads 0; therefore, the device should be discarded when the counter reads 0.
Do not remove the QNASL Nasal Aerosol canister from the actuator. The QNASL Nasal Aerosol canister should only be used with the QNASL Nasal Aerosol actuator and the actuator should not be used with any other drug product.
CONTENTS UNDER PRESSURE
Do not puncture. Do not store near heat or open flame. Do not expose to temperatures higher than 49°C (120°F) as this may cause bursting of the canister. Never throw the device into a fire or an incinerator.
Store at 25°C (77°F); excursions are permitted between 15° and 30°C (59° and 86°F).
Keep out of reach of children.
17 PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling accompanying the product.
17.1 Local Nasal Effects
Patients should be informed that treatment with QNASL Nasal Aerosol may lead to adverse reactions, including epistaxis, nasal ulceration, and nasal discomfort. Candida infection may also occur with treatment with QNASL Nasal Aerosol. In addition, nasal beclomethasone dipropionate products are known to be associated with nasal septal perforation and impaired wound healing. Patients who have experienced recent nasal ulcers, nasal surgery, or nasal trauma should not use QNASL Nasal Aerosol until healing has occurred [see Warnings and Precautions (5.1)].
17.2 Cataracts and Glaucoma
Patients should be informed that glaucoma and cataracts are associated with nasal and inhaled corticosteroid use. Patients should inform their health care providers if a change in vision is noted while using QNASL Nasal Aerosol [see Warnings and Precautions (5.2)].
17.3 Hypersensitivity Reactions Including Anaphylaxis
Hypersensitivity reactions including anaphylaxis, angioedema, urticaria, and rash have been reported following administration of beclomethasone dipropionate nasally administered and inhalationally administered products. Angioedema, urticaria, and rash have been reported following administration of QNASL Nasal Aerosol. If any such reactions occur, patients should discontinue use of QNASL Nasal Aerosol [see Warnings and Precautions (5.3)].
17.4 Immunosuppression
Patients who are on immunosuppressant doses of corticosteroids should be warned to avoid exposure to chickenpox or measles and, if exposed, to consult their physician without delay. Patients should be informed of potential worsening of existing tuberculosis; fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex [see Warnings and Precautions (5.4)].
17.5 Use Daily for Best Effect
Patients should use QNASL Nasal Aerosol on a regular, once-daily basis for optimal effect. QNASL Nasal Aerosol may not have an immediate effect on rhinitis symptoms. The patient should not increase the prescribed dosage but should contact their physician if symptoms do not improve or if the condition worsens.
17.6 Keep Spray Out of Eyes or Mouth
Patients should be informed to avoid spraying QNASL Nasal Aerosol in their eyes or mouth.
Teva Respiratory, LLC
Horsham, PA 19044 USA
©2012, Teva Respiratory, LLC. All rights reserved.
QNASL is a trademark of Teva Respiratory, LLC.
Manufactured for Teva Respiratory, LLC
Horsham, PA 19044
By: 3M Drug Delivery Systems
Northridge, CA 91324
Rev. 06/12
AND INSTRUCTIONS FOR USE LEAFLET
PATIENT INFORMATION
QNASL (kyoo nay' zel)
(beclomethasone dipropionate)
Nasal Aerosol
For Intranasal Use Only
Read this Patient Information before you start using QNASL Nasal Aerosol and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
What is QNASL Nasal Aerosol ?
QNASL Nasal Aerosol is a prescription medicine that treats seasonal nasal and year-round nasal allergy symptoms in adults and adolescents 12 years of age and older.
QNASL Nasal Aerosol contains beclomethasone dipropionate, which is a man-made (synthetic) corticosteroid. Corticosteroids are natural substances found in the body that reduce inflammation. When you spray QNASL Nasal Aerosol into your nose, it may help reduce the nasal symptoms of allergic rhinitis (inflammation of the lining of the nose), such as stuffy nose, runny nose, itching, and sneezing.
It is not known if QNASL Nasal Aerosol is safe and effective in children under 12 years of age.
Who should not use QNASL Nasal Aerosol?
Do not use QNASL Nasal Aerosol if you are allergic to beclomethasone dipropionate or any of the ingredients in QNASL Nasal Aerosol. See the end of this Patient Information leaflet for a complete list of ingredients in QNASL Nasal Aerosol.
What should I tell my healthcare provider before using QNASL Nasal Aerosol?
Before you use QNASL Nasal Aerosol, tell your healthcare provider if you:
- Have had recent nose problems such as nasal sores, nasal surgery, or a nasal injury
- Have or have had eye problems, such as increased pressure in your eye (glaucoma) or cataracts
- Have tuberculosis or any untreated fungal, bacterial, or viral infections, or eye infections caused by herpes
- Have not had or been vaccinated for chickenpox or measles
- Are pregnant or plan to become pregnant. It is not known if QNASL Nasal Aerosol will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant
- Are breastfeeding or plan to breastfeed. It is not known if QNASL Nasal Aerosol passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you are using QNASL Nasal Aerosol
Tell your healthcare provider about all of the medications you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
QNASL Nasal Aerosol and other medications may affect each other and cause side effects. QNASL Nasal Aerosol may affect the way other medications work, and other medications may affect the way QNASL Nasal Aerosol works.
Especially tell your healthcare provider if you take other corticosteroid medicines.
Ask your healthcare provider for a list of these medicines if you are not sure.
Know the medications you take. Keep a list of your medications with you to show your healthcare provider and pharmacist when a new medication is prescribed.
How should I use QNASL Nasal Aerosol?
- Read the Instructions for Use at the end of this leaflet for specific information about the right way to use QNASL Nasal Aerosol
- QNASL Nasal Aerosol is for use in the nose only. Do not spray it in your eyes or mouth
- Use QNASL Nasal Aerosol exactly as your healthcare provider tells you to use it. Do not use more of your medicine or take it more often than your healthcare provider tells you
- QNASL Nasal Aerosol must be primed before you use it for the first time and if you do not use it for 7 or more days in a row. Do not prime your QNASL Nasal Aerosol every day
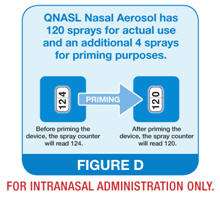
- Your QNASL Nasal Aerosol has a spray counter which should read 120 sprays after your 4 initial priming sprays
- Do not use your QNASL Nasal Aerosol after the spray counter reads 0. You may not get the right amount of medicine
- The usual dose of QNASL Nasal Aerosol is 2 sprays in each nostril, 1 time a day. You should not use more than a total of 4 sprays per day
- You will get the best results if you keep using QNASL Nasal Aerosol regularly each day. If your symptoms do not improve or get worse, call your healthcare provider
What are the possible side effects of QNASL Nasal Aerosol?
QNASL Nasal Aerosol may cause serious side effects, including:
- Nose bleeds or nasal ulcers. Your healthcare provider should check the inside of your nose (nasal mucosa) while you take QNASL Nasal Aerosol for problems. Talk to your healthcare provider if you have nose bleeds or nasal ulcers
- Thrush (candida), a fungal infection in your nose, mouth, or throat. Tell your healthcare provider if you have any redness or white colored patches in your mouth or throat
- Slow wound healing. You should not use QNASL Nasal Aerosol until your nose has healed if you have a sore in your nose, you have had surgery on your nose, or your nose has been injured
- Eye problems such as glaucoma and cataracts. If you have a history of glaucoma or cataracts or have a family history of eye problems, you should have regular eye exams while you use QNASL Nasal Aerosol
-
Adrenal insufficiency. Adrenal insufficiency is a condition in which the adrenal glands do not make enough steroid hormones. Symptoms of adrenal insufficiency may include:
- Tiredness
- Weakness
- Dizziness
- Nausea
- Vomiting
-
Allergic reactions. Serious allergic reactions can happen in people taking QNASL Nasal Aerosol. Stop using QNASL Nasal Aerosol and call your healthcare provider right away or get emergency medical help if you have:
- Shortness of breath or trouble breathing
- Skin rash, redness, or swelling
- Severe itching
- Swelling of your lips, tongue or face
-
Immune system problems that may increase your risk of infections. You are more likely to get infections if you take medicines that may weaken your body's ability to fight infection. Avoid contact with people who have contagious diseases such as chicken pox or measles while you use QNASL Nasal Aerosol. Symptoms of an infection may include:
- Fever
- Pain
- Aches
- Chills
- Feeling tired
- Nausea
- Vomiting
- Slowed growth in children. A child's growth should be checked regularly while using QNASL Nasal Aerosol
The most common side effects with QNASL Nasal Aerosol include:
- Nasal discomfort
- Nose bleeds
- Headache
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of QNASL Nasal Aerosol. For more information, ask your healthcare provider or pharmacist.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store QNASL Nasal Aerosol?
- Store QNASL Nasal Aerosol at room temperature 59˚F to 86˚F (15˚C to 30˚C)
- Do not puncture the QNASL Nasal Aerosol canister
- Do not store the QNASL Nasal Aerosol canister near heat or a flame. Temperatures above 120˚F (49˚C) may cause the canister to burst
- Do not throw the QNASL Nasal Aerosol canister into a fire or an incinerator
- Safely throw away medicine that is out of date or no longer needed
Keep QNASL Nasal Aerosol and all medicines out of the reach of children.
General information about the safe and effective use of QNASL Nasal Aerosol
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use QNASL Nasal Aerosol for a condition for which it was not prescribed. Do not give QNASL Nasal Aerosol to other people, even if they have the same symptoms that you have. It may harm them.
This Patient Information summarizes the most important information about QNASL Nasal Aerosol. If you would like more information, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about QNASL Nasal Aerosol that is written for health professionals.
For more information, go to www.QNASL.com or call 1-855-55-QNASL (1-855-557-6275).
What should I know about allergic rhinitis?
"Rhinitis" means inflammation of the lining of the nose. Allergic rhinitis is sometimes called "hay fever." Allergic rhinitis can be caused by allergies to pollen, animal dander, house dust mites, mold spores, and other things. If you have allergic rhinitis, your nose becomes stuffy, runny, and itchy. You may also sneeze a lot. You may also have red, itchy, watery eyes or an itchy throat; or blocked, itchy ears.
What are the ingredients in QNASL Nasal Aerosol?
Active ingredient: beclomethasone dipropionate
Inactive ingredient: propellant HFA-134a and ethanol
INSTRUCTIONS FOR USE
QNASL (kyoo nay' zel)
(beclomethasone dipropionate)
Nasal Aerosol
Read these Instructions for Use for QNASL Nasal Aerosol before you start using it and each time you get a refill. There may be new information. This leaflet does not take the place of talking to your healthcare provider about your medical condition or treatment.
Note: For Use in the Nose Only.
- Do not spray QNASL Nasal Aerosol in your eyes or directly onto your nasal septum (the wall between your 2 nostrils)
The parts of your QNASL Nasal Aerosol
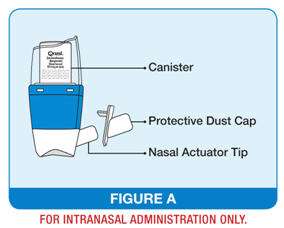
The QNASL Nasal Aerosol device comes as a canister that fits into a nasal actuator with a built-in spray counter and protective dust cap. (See Figure A)
- Do not use the QNASL Nasal Aerosol actuator with a canister of medicine from any other inhaler
- Do not use the QNASL Nasal Aerosol canister with an actuator from any other inhaler
- Do not remove the QNASL Nasal Aerosol canister from the actuator
Priming your QNASL Nasal Aerosol for Use
- Remove your QNASL Nasal Aerosol device from its package
- Your QNASL Nasal Aerosol device must be primed before you use it for the first time or if it has not been used for more than 7 days in a row
- Remove the protective dust cap from the device
- Hold the nasal actuator upright between your thumb and forefinger (index finger). The canister should be on top and the white nasal actuator tip on bottom (See Figure B)

- If you have never used your QNASL Nasal Aerosol device before, spray it 4 times into the air, away from your eyes and face, by pressing down fully on the top of the canister 4 times (See Figure C). Your QNASL Nasal Aerosol device is now ready to use
- After the first time you prime your QNASL Nasal Aerosol device, the spray counter should read 120 (See Figure D)
- Do not prime your QNASL Nasal Aerosol device every day
- If you have used your QNASL Nasal Aerosol device before, but it has not been used in more than 7 days, it must be reprimed. To reprime your QNASL Nasal Aerosol device, spray 2 times into the air, away from your eyes and face, by pressing down fully on the top of the canister 2 times. Your QNASL Nasal Aerosol device is now ready to use
Using Your QNASL Nasal Aerosol Device
Step 1: Blow your nose to clear your nostrils.
Step 2: Remove the protective dust cap from your QNASL Nasal Aerosol device.
Step 3: Inspect the nasal actuator tip to confirm it is clear of foreign objects.
Step 4: Hold your QNASL Nasal Aerosol device upright and insert the nasal actuator tip into one nostril (See Figure E).
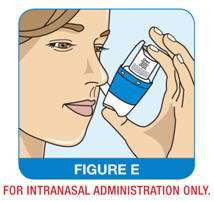
Step 5: Point the QNASL Nasal Aerosol device slightly away from the wall between your nostrils (nasal septum) while holding your other nostril closed (See Figure F).

Step 6: Hold your breath and press down firmly and completely on the canister to release 1 spray (See Figure G). Continue to hold your breath for 5 seconds after releasing the spray and then breathe out slowly through your mouth. Take the QNASL Nasal Aerosol device out of your nostril.
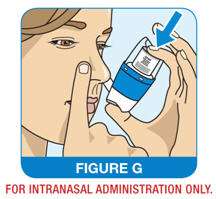
Step 7: Repeat steps 3-6 for the second spray in the same nostril.
Step 8: Repeat steps 3-7 for your other nostril.
Step 9: You should not blow your nose for the next 15 minutes.
Note: The spray counter will count down each time there is a spray released from your QNASL Nasal Aerosol device.
Step 10: Clean and store your device. See "Cleaning Your QNASL Nasal Aerosol device."
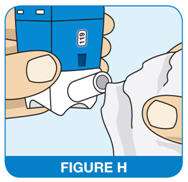
Cleaning Your QNASL Nasal Aerosol device
- Wipe the nasal actuator tip with a clean, dry tissue or cloth (See Figure H)
- Do not wash o r put any part of the QNASL Nasal Aerosol canister or actuator in water
- Replace the protective dust cap
- Keep your device clean and dry at all times
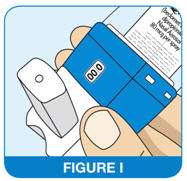
How to know when to stop using your QNASL Aerosol device
- The QNASL Nasal Aerosol device has a spray counter, which is there to let you know how many sprays of medicine you have left
- Do not use your QNASL Nasal Aerosol device when 0 is shown in the spray counter window (See Figure I).
- Throw away your QNASL Nasal Aerosol device when the spray counter reaches 0
- Do not throw your QNASL Nasal Aerosol canister into a fire or an incinerator
- Talk with your health care provider before your supply of QNASL Nasal Aerosol runs out to see if you should get a refill
This PPI and Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for: Teva Respiratory, LLC
Horsham, PA 19044
By: 3M Drug Delivery Systems
Northridge, CA 91324
©2013 Teva Respiratory, LLC
All rights reserved.
QNASL is a registered trademark of Teva Respiratory, LLC
Rev. 05/13 PE 2670
PRINCIPal DISPLAY PANEL, Part 1 of 2
PRINCIPal DISPLAY PANEL, Part 2 of 2
Qnasl 80 mcg Nasal Aerosol 120 Metered Sprays Carton Text
NDC 59310-210-12
QnaslTM
(beclomethasone
dipropionate)
Nasal Aerosol
80 mcg per spray
For Intranasal Use with
Qnasl Actuator Only
Rx only
120 Metered Sprays
8.7g Net Contents
TEVA
QNASLbeclomethasone dipropionate AEROSOL, METERED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||