Propranolol Hydrochloride and Hydrochlorothiazide
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
- PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE DESCRIPTION
Propranolol hydrochloride and hydrochlorothiazide tablets, USP for oral administration combine two antihypertensive agents: propranolol hydrochloride, USP, a beta-adrenergic blocking agent, and hydrochlorothiazide, USP, a thiazide diuretic-antihypertensive. Propranolol hydrochloride and hydrochlorothiazide tablets, 40/25 contain 40 mg propranolol hydrochloride and 25 mg hydrochlorothiazide; propranolol hydrochloride and hydrochlorothiazide tablets, 80/25 contain 80 mg propranolol hydrochloride and 25 mg hydrochlorothiazide.
Propranolol hydrochloride is a synthetic beta-adrenergic receptor-blocking agent chemically described as 1-(Isopropylamino)-3-(1-naphthyloxy)-2-propanol hydrochloride. Its structural formula is:
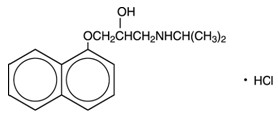
Propranolol hydrochloride, USP is a stable, white, crystalline solid which is readily soluble in water and ethanol. Its molecular weight is 295.81.
Hydrochlorothiazide, USP is a white, or practically white, practically odorless, crystalline powder. It is slightly soluble in water; freely soluble in sodium hydroxide solution; sparingly soluble in methanol; insoluble in ether, chloroform, benzene, and dilute mineral acids. Its chemical name is: 6-Chloro-3,4-dihydro-2H-1,2,4-benzothiadiazine-7-sulfonamide 1,1-dioxide. Its structural formula is:
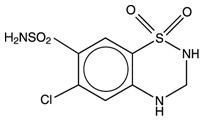
Propranolol hydrochloride and hydrochlorothiazide tablets, 40 mg/25 mg and 80 mg/25 mg are for oral administration and contain the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, magnesium stearate, microcrystalline cellulose, pregelatinized corn starch and sodium lauryl sulfate.
CLINICAL PHARMACOLOGY
Propranolol Hydrochloride
Propranolol hydrochloride is a nonselective beta-adrenergic receptor blocking agent possessing no other autonomic nervous system activity. It specifically competes with beta-adrenergic receptor stimulating agents for available receptor sites. When access to beta-receptor sites is blocked by propranolol, the chronotropic, inotropic, and vasodilator responses to beta-adrenergic stimulation are decreased proportionately.
Propranolol is almost completely absorbed from the gastrointestinal tract, but a portion is immediately metabolized by the liver on its first pass through the portal circulation.
Peak effect occurs in one to one-and-one-half hours. The biologic half-life is approximately 4 hours. Propranolol is not significantly dialyzable. There is no simple correlation between dose or plasma level and therapeutic effect, and the dose-sensitivity range, as observed in clinical practice, is wide. The principal reason for this is that sympathetic tone varies widely between individuals. Since there is no reliable test to estimate sympathetic tone or to determine whether total beta-blockade has been achieved, proper dosage requires titration.
The mechanism of the antihypertensive effect of propranolol has not been established. Among the factors that may be involved in contributing to the antihypertensive action are (1) decreased cardiac output, (2) inhibition of renin release by the kidneys, and (3) diminution of tonic sympathetic nerve outflow from vasomotor centers in the brain. Although total peripheral resistance may increase initially, it readjusts to, or below, the pretreatment level with chronic use. Effects on plasma volume appear to be minor and somewhat variable. Propranolol has been shown to cause a small increase in serum potassium concentration when used in the treatment of hypertensive patients. Propranolol hydrochloride decreases heart rate, cardiac output, and blood pressure.
Beta-receptor blockade can be useful in conditions in which, because of pathologic or functional changes, sympathetic activity is detrimental to the patient. But there are also situations in which sympathetic stimulation is vital. For example, in patients with severely damaged hearts, adequate ventricular function is maintained by virtue of sympathetic drive, which should be preserved. In the presence of AV block greater than first degree, beta-blockade may prevent the necessary facilitating effect of sympathetic activity on conduction. Beta-blockade results in bronchial constriction by interfering with adrenergic bronchodilator activity, which should be preserved in patients subject to bronchospasm.
The proper objective of beta-blockade therapy is to decrease adverse sympathetic stimulation, but not to the degree that may impair necessary sympathetic support.
Hydrochlorothiazide
Hydrochlorothiazide is a benzothiadiazine (thiazide) diuretic closely related to chlorothiazide. The mechanism of the antihypertensive effect of the thiazides is unknown. Thiazides do not affect normal blood pressure.
Thiazides affect the renal tubular mechanism of electrolyte reabsorption. At maximal therapeutic dosage, all thiazides are approximately equal in their diuretic potency.
Thiazides increase excretion of sodium and chloride in approximately equivalent amounts. Natriuresis causes a secondary loss of potassium and bicarbonate. Onset of diuretic action of hydrochlorothiazide occurs in 2 hours, and the peak effect in about 4 hours. Its action persists for approximately 6 to 12 hours. Thiazides are eliminated rapidly by the kidney.
PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
Propranolol hydrochloride and hydrochlorothiazide tablets are indicated in the management of hypertension.
This fixed combination is not indicated for initial therapy of hypertension. Hypertension requires therapy titrated to the individual patient. If the fixed combination represents the dosage so determined, its use may be more convenient in patient management.
PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
Propranolol Hydrochloride
Propranolol is contraindicated in 1) cardiogenic shock; 2) sinus bradycardia and greater than first-degree block; 3) bronchial asthma; 4) congestive heart failure (see WARNINGS) unless the failure is secondary to a tachyarrhythmia treatable with propranolol.
Hydrochlorothiazide
Hydrochlorothiazide is contraindicated in patients with anuria or hypersensitivity to this or other sulfonamide-derived drugs.
WARNINGS
Propranolol Hydrochloride
Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions, have been associated with the administration of propranolol and hydrochlorothiazide (see ADVERSE REACTIONS).
Cardiac Failure
Sympathetic stimulation is a vital component supporting circulatory function in congestive heart failure, and inhibition with beta-blockade always carries the potential hazard of further depressing myocardial contractility and precipitating cardiac failure. Propranolol acts selectively without abolishing the inotropic action of digitalis on the heart muscle (i.e., that of supporting the strength of myocardial contractions). In patients already receiving digitalis, the positive inotropic action of digitalis may be reduced by propranolol’s negative inotropic effect.
Patients Without a History of Heart Failure
Continued depression of the myocardium over a period of time can, in some cases, lead to cardiac failure. In rare instances, this has been observed during propranolol therapy. Therefore, at the first sign or symptom of impending cardiac failure, patients should be fully digitalized and/or given additional diuretic, and the response observed closely: a) if cardiac failure continues, despite adequate digitalization and diuretic therapy, propranolol therapy should be withdrawn (gradually, if possible); b) if tachyarrhythmia is being controlled, patients should be maintained on combined therapy and the patient closely followed until threat of cardiac failure is over.
There have been reports of exacerbation of angina and, in some cases, myocardial infarction following abrupt discontinuation of propranolol therapy. Therefore, when discontinuance of propranolol is planned, the dosage should be gradually reduced and the patient should be carefully monitored. In addition, when propranolol is prescribed for angina pectoris, the patient should be cautioned against interruption or cessation of therapy without the physician’s advice. If propranolol therapy is interrupted and exacerbation of angina occurs, it usually is advisable to reinstitute propranolol therapy and take other measures appropriate for the management of unstable angina pectoris. Since coronary artery disease may be unrecognized, it may be prudent to follow the above advice in patients considered at risk of having occult atherosclerotic heart disease, who are given propranolol for other indications.
Nonallergic Bronchospasm (e.g., chronic bronchitis, emphysema)
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS. Propranolol should be administered with caution since it may block bronchodilation produced by endogenous and exogenous catecholamine stimulation of beta-receptors.
Major Surgery
Chronically administered beta-blocking therapy should not be routinely withdrawn prior to major surgery, however the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Diabetes and Hypoglycemia
Beta-adrenergic blockade may prevent the appearance of certain premonitory signs and symptoms (pulse rate and pressure changes) of acute hypoglycemia in labile insulin-dependent diabetes. In these patients, it may be more difficult to adjust the dosage of insulin. Hypoglycemic attack may be accompanied by a precipitous elevation of blood pressure in patients on propranolol.
Propranolol therapy, particularly in infants and children, diabetic or not, has been associated with hypoglycemia especially during fasting as in preparation for surgery. Hypoglycemia also has been found after this type of drug therapy and prolonged physical exertion and has occurred in renal insufficiency, both during dialysis and sporadically, in patients on propranolol.
Acute increases in blood pressure have occurred after insulin-induced hypoglycemia in patients on propranolol.
Thyrotoxicosis
Beta-blockade may mask certain clinical signs of hyperthyroidism. Therefore, abrupt withdrawal of propranolol may be followed by an exacerbation of symptoms of hyperthyroidism, including thyroid storm. Propranolol may change thyroid-function tests, increasing T4 and reverse T3, and decreasing T3.
Wolff-Parkinson-White Syndrome
Several cases have been reported in which, after propranolol, the tachycardia was replaced by a severe bradycardia requiring a demand pacemaker. In one case this resulted after an initial dose of 5 mg propranolol.
Skin Reactions
Cutaneous reactions, including Stevens-Johnson Syndrome, toxic epidermal necrolysis, exfoliative dermatitis, erythema multiforme, and urticaria, have been reported with use of propranolol (see ADVERSE REACTIONS).
Hydrochlorothiazide
Thiazides should be used with caution in severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. In patients with impaired renal function, cumulative effects of the drug may develop.
Thiazides should also be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte balance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic-blocking drugs.
Sensitivity reactions may occur in patients with a history of allergy or bronchial asthma. The possibility of exacerbation or activation of systemic lupus erythematosus has been reported.
Acute Myopia and Secondary Angle Closure Glaucoma
Hydrochlorothiazide, a sulfonamide, can cause an idiosyncratic reaction, resulting in acute transient myopia and acute angle closure glaucoma. Symptoms include acute onset of decreased visual acuity or ocular pain and typically occur within hours to weeks of drug initiation. Untreated acute angle closure glaucoma can lead to permanent vision loss. The primary treatment is to discontinue hydrochlorothiazide as rapidly as possible. Prompt medical or surgical treatments may need to be considered if the intraocular pressure remains uncontrolled. Risk factors for developing acute angle closure glaucoma may include a history of sulfonamide or penicillin allergy.
PRECAUTIONS
General
Propranolol Hydrochloride
Propranolol should be used with caution in patients with impaired hepatic or renal function. Propranolol hydrochloride and hydrochlorothiazide tablets are not indicated for the treatment of hypertensive emergencies.
Risk of Anaphylactic Reaction
While taking beta-blockers, patients with a history of severe anaphylactic reaction to a variety of allergens may be more reactive to repeated challenge, either accidental, diagnostic, or therapeutic. Such patients may be unresponsive to the usual doses of epinephrine used to treat allergic reaction.
Hydrochlorothiazide
All patients receiving thiazide therapy should be observed for clinical signs of fluid or electrolyte imbalance, namely hyponatremia, hypochloremic alkalosis, and hypokalemia. Serum and urine electrolyte determinations are particularly important when the patient is vomiting excessively or receiving parenteral fluids. Medication such as digitalis may also influence serum electrolytes. Warning signs, irrespective of cause, are: dryness of mouth, thirst, weakness, lethargy, drowsiness, restlessness, muscle pains or cramps, muscular fatigue, hypotension, oliguria, tachycardia, and gastrointestinal disturbances such as nausea and vomiting.
Hypokalemia may develop, especially with brisk diuresis or when severe cirrhosis is present.
Interference with adequate oral electrolyte intake will also contribute to hypokalemia. Hypokalemia can sensitize or exaggerate the response of the heart to the toxic effects of digitalis (e.g., increased ventricular irritability).
Hypokalemia may be avoided or treated by use of potassium supplements or foods with a high potassium content.
Any chloride deficit is generally mild, and usually does not require specific treatment except under extraordinary circumstances (as in liver or renal disease). Dilutional hyponatremia may occur in edematous patients in hot weather; appropriate therapy is water restriction rather than administration of salt, except in rare instances when the hyponatremia is life threatening. In actual salt depletion, appropriate replacement is the therapy of choice.
Hyperuricemia may occur or frank gout may be precipitated in certain patients receiving thiazide therapy.
Diabetes mellitus which has been latent may become manifest during thiazide administration.
The antihypertensive effects of the drug may be enhanced in the postsympathectomy patient.
If progressive renal impairment becomes evident, consider withholding or discontinuing diuretic therapy.
Calcium excretion is decreased by thiazides. Pathologic changes in the parathyroid gland with hypercalcemia and hypophosphatemia have been observed in a few patients on prolonged thiazide therapy. The common complications of hyperparathyroidism, such as renal lithiasis, bone resorption, and peptic ulceration, have not been seen.
Information for Patients
Beta-adrenoreceptor blockade can cause reduction of intraocular pressure. Patients should be told that propranolol hydrochloride and hydrochlorothiazide may interfere with the glaucoma screening test. Withdrawal may lead to a return of increased intraocular pressure.
Laboratory Tests
Propranolol Hydrochloride
Elevated blood urea levels in patients with severe heart disease, elevated serum transaminase, alkaline phosphatase, lactate dehydrogenase.
Hydrochlorothiazide
Periodic determination of serum electrolytes to detect possible electrolyte imbalance should be performed at appropriate intervals.
Drug/Drug Interactions
Propranolol Hydrochloride
Patients receiving catecholamine-depleting drugs such as reserpine should be closely observed if propranolol hydrochloride and hydrochlorothiazide is administered. The added catecholamine-blocking action may produce an excessive reduction of resting sympathetic nervous activity, which may result in hypotension, marked bradycardia, vertigo, syncopal attacks, or orthostatic hypotension.
Caution should be exercised when patients receiving a beta-blocker are administered a calcium-channel blocking drug, especially intravenous verapamil, for both agents may depress myocardial contractility or atrioventricular conduction. On rare occasions, the concomitant intravenous use of a beta-blocker and verapamil has resulted in serious adverse reactions, especially in patients with severe cardiomyopathy, congestive heart failure, or recent myocardial infarction.
Both digitalis glycosides and beta-blockers slow atrioventricular conduction and decrease heart rate. Concomitant use can increase the risk of bradycardia.
Blunting of the antihypertensive effect of beta-adrenoceptor blocking agents by nonsteroidal anti-inflammatory drugs has been reported.
Hypotension and cardiac arrest have been reported with the concomitant use of propranolol and haloperidol.
Aluminum hydroxide gel greatly reduces intestinal absorption of propranolol.
Alcohol, when used concomitantly with propranolol, may increase plasma levels of propranolol.
Phenytoin, phenobarbitone, and rifampin accelerate propranolol clearance.
Chlorpromazine, when used concomitantly with propranolol, results in increased plasma levels of both drugs.
Antipyrine and lidocaine have reduced clearance when used concomitantly with propranolol.
Thyroxine may result in a lower than expected T3 concentration when used concomitantly with propranolol.
Cimetidine decreases the hepatic metabolism of propranolol, delaying elimination and increasing blood levels.
Theophylline clearance is reduced when used concomitantly with propranolol.
Hydrochlorothiazide
Thiazide drugs may increase the responsiveness to tubocurarine.
Thiazides may decrease arterial responsiveness to norepinephrine. This diminution is not sufficient to preclude effectiveness of the pressor agent for therapeutic use.
Insulin requirements in diabetic patients may be increased, decreased, or unchanged.
Hypokalemia may develop during concomitant use of corticosteroids or ACTH.
Drug/Laboratory Test Interactions
Hydrochlorothiazide
Thiazides may decrease serum PBI levels without signs of thyroid disturbance.
Thiazides should be discontinued before carrying out tests for parathyroid function (see PRECAUTIONS: General).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Combinations of propranolol and hydrochlorothiazide have not been evaluated for carcinogenic or mutagenic potential or for potential to adversely affect fertility.
Propranolol Hydrochloride
In dietary administration studies in which mice and rats were treated with propranolol for up to 18 months at doses of up to 150 mg/kg/day, there was no evidence of drug-related tumorigenesis. In a study in which both male and female rats were exposed to propranolol in their diets at concentrations of up to 0.05%, from 60 days prior to mating and throughout pregnancy and lactation for two generations, there were no effects on fertility. Based on differing results from Ames Tests performed by different laboratories, there is equivocal evidence for a genotoxic effect of propranolol in bacteria (S. typhimurium strain TA 1538).
Hydrochlorothiazide
Two-year feeding studies in mice and rats conducted under the auspices of the National Toxicology Program (NTP) uncovered no evidence of a carcinogenic potential of hydrochlorothiazide in female mice (at doses of up to approximately 600 mg/kg/day) or in male and female rats (at doses of up to approximately 100 mg/kg/day). The NTP, however, found equivocal evidence for hepatocarcinogenicity in male mice.
Hydrochlorothiazide was not genotoxic in vitro in the Ames bacterial mutagen assay (S. typhimurium strains TA 98, TA 100, TA 1535, TA 1537 and TA 1538) or in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations. Nor was it genotoxic in vivo in assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained in the in vitro CHO Sister Chromatid Exchange (clastogenicity), Mouse Lymphoma Cell (mutagenicity) and Aspergillus nidulans non-disjunction assays.
Hydrochlorothiazide had no adverse effects on the fertility of mice and rats of either sex in studies wherein these species were exposed, via their diet, to doses of up to 100 mg/kg and 4 mg/kg, respectively, prior to mating and throughout gestation.
Pregnancy
Teratogenic Effects. Pregnancy Category C
Combinations of propranolol and hydrochlorothiazide have not been evaluated for effects on pregnancy in animals. Nor are there adequate and well controlled studies of propranolol, hydrochlorothiazide or the combination in pregnant women. Propranolol and hydrochlorothiazide should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Propranolol Hydrochloride
In a series of reproduction and developmental toxicology studies, propranolol was given to rats by gavage or in the diet throughout pregnancy and lactation. At doses of 150 mg/kg/day (> 30 times the dose of propranolol contained in the maximum recommended human daily dose of propranolol hydrochloride and hydrochlorothiazide), but not at doses of 80 mg/kg/day, treatment was associated with embryotoxicity (reduced litter size and increased resorption sites) as well as neonatal toxicity (deaths). Propranolol also was administered (in the feed) to rabbits (throughout pregnancy and lactation) at doses as high as 150 mg/kg/day (> 45 times the dose of propranolol contained in the maximum recommended daily human dose of propranolol hydrochloride and hydrochlorothiazide). No evidence of embryo or neonatal toxicity was noted.
Intrauterine growth retardation, small placentas, and congenital abnormalities have been reported in human neonates whose mothers received propranolol during pregnancy. Neonates whose mothers received propranolol at parturition have exhibited bradycardia, hypoglycemia and/or respiratory depression. Adequate facilities for monitoring these infants at birth should be available.
Hydrochlorothiazide
Studies in which hydrochlorothiazide was orally administered to pregnant mice and rats at doses of up to 3000 and 1000 mg/kg/day, respectively, provided no evidence of harm to the fetus.
Thiazides cross the placental barrier and appear in cord blood. The use of thiazides in pregnant women requires that the anticipated benefit be weighed against possible hazards to the fetus. These hazards include fetal or neonatal jaundice, thrombocytopenia, and possibly other adverse reactions that have occurred in the adult.
Nursing Mothers
Propranolol Hydrochloride
Propranolol is excreted in human milk. Caution should be exercised when propranolol hydrochloride and hydrochlorothiazide is administered to a nursing woman.
Hydrochlorothiazide
Thiazides appear in breast milk. If the use of the drug is deemed essential, the patient should stop nursing.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Clinical studies of propranolol hydrochloride and hydrochlorothiazide did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
The following adverse reactions have been observed, but there is not enough systematic collection of data to support an estimate of their frequency. Within each category, adverse reactions are listed in decreasing order of severity. Although many side effects are mild and transient, some require discontinuation of therapy.
Propranolol Hydrochloride
Cardiovascular: Congestive heart failure; hypotension; intensification of AV block; bradycardia; thrombocytopenic purpura; arterial insufficiency, usually of the Raynaud type; paresthesia of hands.
Central Nervous System: Reversible mental depression progressing to catatonia; mental depression manifested by insomnia, lassitude, weakness, fatigue; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, decreased performance on neuropsychometrics; hallucinations; visual disturbances; vivid dreams; light-headedness. Total daily doses above 160 mg (when administered as divided doses of greater than 80 mg each) may be associated with an increased incidence of fatigue, lethargy, and vivid dreams.
Gastrointestinal: Mesenteric arterial thrombosis; ischemic colitis; nausea, vomiting, epigastric distress, abdominal cramping, diarrhea, constipation.
Allergic: Hypersensitivity reactions, including anaphylactic/anaphylactoid reactions; laryngospasm and respiratory distress; pharyngitis and agranulocytosis; fever combined with aching and sore throat; erythematous rash.
Respiratory: Bronchospasm.
Hematologic: Agranulocytosis; non-thrombocytopenic purpura; thrombocytopenic purpura.
Autoimmune: In extremely rare instances, systemic lupus erythematosus has been reported.
Miscellaneous: Male impotence. Alopecia, LE-like reactions, psoriasiform rashes, dry eyes, and Peyronie’s disease have been reported rarely. Oculomucocutaneous reactions involving the skin, serous membranes, and conjunctivae reported for a beta-blocker (practolol) have not been associated with propranolol.
Skin: Stevens-Johnson Syndrome; toxic epidermal necrolysis; exfoliative dermatitis; erythema multiforme; urticaria.
Hydrochlorothiazide
Cardiovascular: Orthostatic hypotension (may be aggravated by alcohol, barbiturates or narcotics).
Central Nervous System: Dizziness, vertigo, headache, xanthopsia, paresthesias.
Gastrointestinal: Pancreatitis; jaundice (intrahepatic cholestatic jaundice); sialadenitis; anorexia, nausea, vomiting, gastric irritation, cramping, diarrhea, constipation.
Hypersensitivity: Anaphylactic reactions; necrotizing angiitis (vasculitis, cutaneous vasculitis); respiratory distress including pneumonitis; fever; urticaria, rash, purpura, photosensitivity.
Hematologic: Aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia.
Skin: Erythema multiforme including Stevens-Johnson Syndrome, exfoliative dermatitis including toxic epidermal necrolysis.
Miscellaneous: Hyperglycemia, glycosuria; hyperuricemia; muscle spasm; weakness; restlessness; transient blurred vision.
Whenever adverse reactions are moderate or severe, thiazide dosage should be reduced or therapy withdrawn.
OVERDOSAGE
The propranolol hydrochloride component may cause bradycardia, cardiac failure, hypotension, or bronchospasm. Propranolol is not significantly dialyzable.
The hydrochlorothiazide component can be expected to cause diuresis. Lethargy of varying degree may appear and may progress to coma within a few hours, with minimal depression of respiration and cardiovascular function, and in the absence of significant serum electrolyte changes or dehydration. The mechanism of central nervous system depression with thiazide overdosage is unknown. Gastrointestinal irritation and hypermotility can occur, temporary elevation of BUN has been reported, and serum electrolyte changes could occur, especially in patients with impairment of renal function.
The oral LD50 dosages in rats and mice for propranolol, hydrochlorothiazide and combined propranolol/hydrochlorothiazide (40/25, 80/25) are 364 to 533 mg/kg, greater than 2750 to 5000 mg/kg, and 538 to 845 mg/kg, respectively.
Treatment
The following measures should be employed:
General: If ingestion is, or may have been, recent, evacuate gastric contents, taking care to prevent pulmonary aspiration.
Bradycardia: Administer atropine (0.25 mg to 1 mg). If there is no response to vagal blockade, administer isoproterenol cautiously.
Cardiac Failure: Digitalization and diuretics.
Hypotension: Vasopressors, e.g., levarterenol or epinephrine.
Bronchospasm: Administer isoproterenol and aminophylline.
Stupor or Coma: Administer supportive therapy as clinically warranted.
Gastrointestinal Effects: Though usually of short duration, these may require symptomatic treatment.
Abnormalities in BUN and/or Serum Electrolytes: Monitor serum electrolyte levels and renal function; institute supportive measures as required individually to maintain hydration, electrolyte balance, respiration, and cardiovascular-renal function.
PROPRANOLOL HYDROCHLORIDE AND HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
The dosage must be determined by individual titration.
Hydrochlorothiazide can be given at doses of 12.5 to 50 mg per day when used alone. The initial dose of propranolol is 80 mg daily, and it may be increased gradually until optimal blood pressure control is achieved. The usual effective dose when used alone is 160 to 480 mg per day.
One propranolol hydrochloride and hydrochlorothiazide tablet twice daily can be used to administer up to 160 mg of propranolol and 50 mg of hydrochlorothiazide. For doses of propranolol greater than 160 mg the combination products are not appropriate, because their use would lead to an excessive dose of the thiazide component.
When necessary, another antihypertensive agent may be added gradually beginning with 50 percent of the usual recommended starting dose to avoid an excessive fall in blood pressure.
HOW SUPPLIED
Propranolol Hydrochloride and Hydrochlorothiazide Tablets, USP are available in the following combinations:
The 40 mg/25 mg tablets contain 40 mg of propranolol hydrochloride, USP and 25 mg of hydrochlorothiazide, USP. Each white round scored tablet is debossed with MYLAN over 731 on one side of the tablet and scored on the other side. They are available as follows:
NDC 0378-0731-01
bottles of 100 tablets
The 80 mg/25 mg tablets contain 80 mg of propranolol hydrochloride, USP and 25 mg of hydrochlorothiazide, USP. Each white round scored tablet is debossed with MYLAN over 347 on one side of the tablet and scored on the other side. They are available as follows:
NDC 0378-0347-01
bottles of 100 tablets
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from moisture, freezing, and excessive heat.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
REVISED JULY 2012
PRAN/HCTZ:R19
PRINCIPAL DISPLAY PANEL - 40 mg/25 mg
NDC 0378-0731-01
Propranolol HCl and
Hydrochlorothiazide
Tablets, USP
40 mg/
25 mg
Rx only 100 Tablets
Each tablet contains:
Propranolol
hydrochloride, USP 40 mg
Hydrochlorothiazide, USP 25 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Protect from moisture, freezing,
and excessive heat.
Usual Adult Dosage: See
accompanying prescribing
information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM0731A7

PRINCIPAL DISPLAY PANEL - 80 mg/25 mg
NDC 0378-0347-01
Propranolol HCl and
Hydrochlorothiazide
Tablets, USP
80 mg/
25 mg
Rx only 100 Tablets
Each tablet contains:
Propranolol
hydrochloride, USP 80 mg
Hydrochlorothiazide, USP 25 mg
Dispense in a tight, light-resistant
container as defined in the USP
using a child-resistant closure.
Keep container tightly closed.
Keep this and all medication
out of the reach of children.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room
Temperature.]
Protect from moisture, freezing,
and excessive heat.
Usual Adult Dosage: See
accompanying prescribing
information.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Mylan.com
RM0347A8

Propranolol Hydrochloride and Hydrochlorothiazidepropranolol hydrochloride and hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Propranolol Hydrochloride and Hydrochlorothiazidepropranolol hydrochloride and hydrochlorothiazide TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||