Propranolol Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- PROPRANOLOL HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- PHARMACODYNAMICS
- INDICATIONS & USAGE
- PROPRANOLOL HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PROPRANOLOL HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- INACTIVE INGREDIENT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PROPRANOLOL HYDROCHLORIDE DESCRIPTION
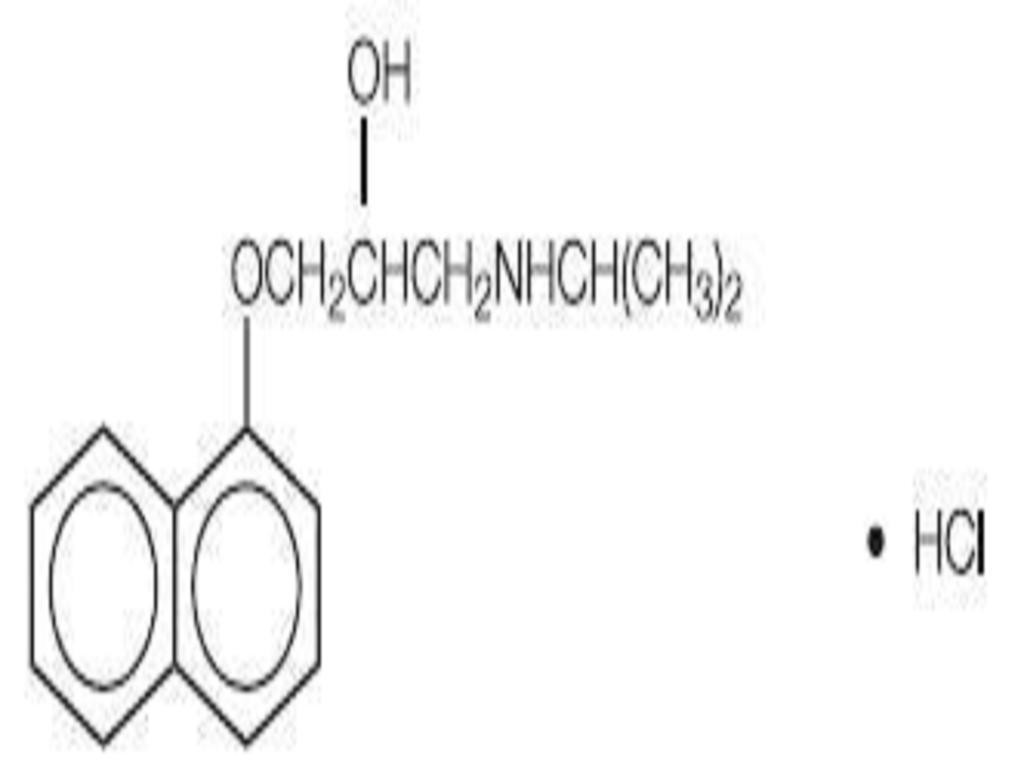
CLINICAL PHARMACOLOGY
GeneralMechanism of Action
PHARMACOKINETICS
AbsorptionDistribution
Metabolism and Elimination
Enantiomers
Special Populations
PRECAUTIONS
Drug Interactions
Drug InteractionsPRECAUTIONS
PHARMACODYNAMICS
HypertensionAngina Pectoris
Atrial Fibrillation
Myocardial Infarction
Migraine
Essential Tremor
Hypertrophic Subaortic Stenosis
Pheochromocytoma
INDICATIONS & USAGE
PROPRANOLOL HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
ADVERSE REACTIONS
ADVERSE REACTIONS
PRECAUTIONS
GeneralClinical Laboratory Tests
Drug Interactions
Drug InteractionsPHARMACOKINETICS AND DRUG METABOLISM
OVERDOSAGE
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
PROPRANOLOL HYDROCHLORIDE ADVERSE REACTIONS
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGS
PHARMACOKINETICS AND DRUG METABOLISM
HOW SUPPLIED
INACTIVE INGREDIENT
ANHYDROUS LACTOSECOLLOIDAL SILICON DIOXIDE
CROSCARMELLOSE SODIUM
D&C YELLOW NO. 10
MAGNESIUM STEARATE
CELLULOSE, MICROCRYSTALLINE
STEARIC ACID
FD&C BLUE NO. 1
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Propranolol HydrochloridePropranolol Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!