Program
Novartis Animal Health US, Inc.
Program Suspension
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
Suspension
The once-a-month oral suspension that controls flea populations.
ct
Novartis Animal Health encourages you to take the time to read this package insert which describes the use of PROGRAM® (lufenuron) Suspension as a convenient monthly flea control treatment. Controlling flea infestations is very important to the health of your pet. After reading this insert, if you have any questions about PROGRAM Suspension, flea control or medical problems associated with flea infestations, consult your veterinarian, who is your pet’s healthcare expert.
| TO ENSURE ADEQUATE ABSORPTION, ALWAYS ADMINISTER PROGRAM SUSPENSION TO CATS IN CONJUNCTION WITH A NORMAL MEAL. |
PROGRAM Suspension is administered by mixing into food. If the dose is not entirely consumed, redose once with the full recommended dose as soon as possible. In multiple cat households, each cat should be treated separately to ensure adequate dosing.
PROGRAM Suspension must be administered monthly, preferably on the same date each month in conjunction with a normal meal. Treatment with PROGRAM Suspension may begin at any time of the year. Treatment should continue until the end of “flea season.” If there is risk of exposure to fleas year-round, then treatment should continue the entire year without interruption. Ask you veterinarian for details concerning your geographic area and the most effective treatment schedule for your pet.
To maximize benefits from the use of PROGRAM Suspension, it is important to treat all cats within a household. All dogs within the household should be treated with PROGRAM Tablets. Fleas can reproduce on untreated cats and dogs and allow infestations to persist.
Mix the entire contents of the appropriate size PROGRAM Suspension pack(s) with about two tablespoons of the cat’s food. Since PROGRAM Suspension is a liquid, it mixes easily with wet food. You can make it a special treat for your cat by mixing it with your cat’s favorite wet food.
The active ingredient in PROGRAM is excreted in high concentrations in the milk, however, no resulting adverse effects have been recognized.
Although other flea species may be found on cats, the cat flea (Ctenocephalides felis) is the predominant flea associated with infestations on cats in the United States. In addition to the common nuisance irritations associated with infestations, fleas can be responsible for medical problems in your pet such as military dermatitis, a skin reaction to flea bites. Also, fleas transmit other parasites, including tapeworms. Controlling flea infestations is important to your pet’s health while also reducing the major and minor annoyances associated with these parasites.
Lufenuron, the active ingredient of PROGRAM Suspension does not kill adult fleas but effectively breaks the flea’s life cycle by inhibiting egg development. The following diagram illustrates the flea’s life cycle and where PROGRAM acts to break this cycle:
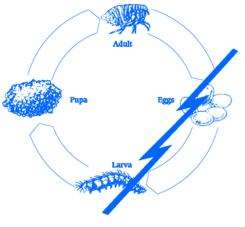
Fleas can be a problem because they reproduce so rapidly. A single female flea may produce up to 2,000 eggs over her lifetime. Eggs hatch and can develop into adults within only three weeks. Adult female fleas feed by ingesting blood from your cat and subsequently lay eggs, which drop off your cat’s coat. Within days, larvae hatch from the eggs and live undetected in your cat’s surroundings such as the carpet, bedding, and other protected areas. Flea larvae spin a cocoon, and when appropriately stimulated, a young adult flea emerges and jumps onto your cat to continue the life cycle. After biting a PROGRAM Suspension treated cat, the female flea ingests lufenuron which is deposited in her eggs. Lufenuron prevents these eggs from hatching or developing into mature adults. This safe and convenient approach to flea control effectively breaks the flea’s life cycle and controls flea populations.
Observe the cat closely to ensure the entire dose has been consumed and then provide its normal meal. Always administer PROGRAM (lufenuron) Suspension to cats in conjunction with a normal meal as food is important for complete absorption of PROGRAM. If your cat does not consume its meal following administration of PROGRAM, try withholding the cat’s food the night before to ensure it eats a meal with PROGRAM.
Some cats have special dietary needs, so be sure to consult your veterinarian before giving your cat any new foods or before withholding food overnight.
PROGRAM Suspension is available in two sizes of packs for oral administration to cats and kittens according to their weight (see Dosage section). The active ingredient of PROGRAM Suspension is lufenuron, a benzoylphenylurea derivative with the following chemical composition: N-(2,5-dichloro-4(1,1,2,3,3,3,-hexafluoropropoxy)-phenylaminocarbonyl)-2,6difluorobenzamide. Benzoylphenylurea compounds, including lufenuron, are classified as insect development inhibitors (IDIs).
As an insect development inhibitor, PROGRAM does not kill adult fleas, but effectively and safely controls flea populations on your pet through a mode of action which breaks the flea’s life cycle at the egg stage.
PROGRAM Suspension is labeled for use in cats and kittens, six weeks of age and older, for the control of flea populations.
PROGRAM Suspension has no effect on adult fleas but acts to immediately break the flea life cycle by preventing eggs from developing into adults. However, pre-existing immature fleas in the cat’s environment may continue to develop and emerge as adults after treatment with PROGRAM Suspension. Based on results of clinical studies, this emergence generally occurs during the first 30-60 days. Therefore, if your pet already has a flea infestation before starting PROGRAM, noticeable results may not be observed until several weeks after dosing. In cooler climates, immature fleas may take longer to complete the life cycle and emerge as adults. To speed control, traditional products that kill adult fleas may be used temporarily with PROGRAM, depending on the severity of the infestation.
If a PROGRAM-treated cat comes in contact with a flea-infested environment, adult fleas may infest the treated animal. However, these adult fleas will be unable to reproduce and will soon die off. Depending on the number of adult fleas, the temporary use of conventional adulticidal insecticides may be used to control these adult fleas. Your veterinarian can recommend the most effective treatment plan for your pet.
To ensure that your pet gets the greatest benefit from PROGRAM Suspension, you must administer the dose once a month in conjunction with a normal meal. If you miss the 30-day interval, give PROGRAM Suspension to your cat immediately and resume your monthly dosing schedule. It is important to treat all cats within the household. All dogs within the household should be treated with PROGRAM Tablets. Fleas can reproduce on untreated dogs and cats and allow infestations to persist.
PROGRAM Suspension is given orally by mixing into food, once a month, at the recommended minimum dosage of 13.6 mg lufenuron per pound (30mg/kg) of body weight.
| Body Weight | Pack Per Month |
Lufenuron Per Pack |
Pack Color |
| Up to 10 lbs | 1 Small | 135 mg | Orange |
| 11 to 20 lbs | 1 Large | 270 mg | Green |
Cats over 20 lbs should receive the appropriate combination of packs.
PROGRAM Suspension will safely and effectively control flea infestations only if administered on a monthly dosing schedule. To help you remember the monthly dosing, use the enclosed reminder stickers on the appropriate dates on your calendar.
The following adverse reactions have been reported in cats after giving PROGRAM: vomiting, depression/lethargy, anorexia (loss of appetite), diarrhea, dyspnea (labored breathing), pruritus (itchy, scratchy skin), and skin disorder.
PROGRAM Suspension is available in two pack sizes (see Dosage section), formulated and color-coded according to the weight of the cat. Both pack sizes are available in color-coded packages of 6 packs per carton.
PROGRAM Suspension should be stored at room temperature between 59° and 77°F (15-25°C).
Keep out of the reach of children.
Manufactured for:
Novartis Animal Health US, Inc.
Greensboro, NC 27408, USA
NADA # 141-026, Approved by FDA
©2008 Novartis Animal Health US, Inc. NAH/PRO-S/PI/2
1/08
Package Label –
135
mg
PROGRAM® (LUFENURON)
Once-a-month oral suspension
For the control of flea populations in cats
Recommended for cats and kittens up to 10 lbs. in weight.
Keep this and all drugs out of the reach of children.
Manufactured for: Novartis Animal Health US, Inc. Greensboro, NC 27408 U.S.A.
NADA # 141-026 Approved by the FDA www.novartisah.com
GIVE WITH A MEAL Net contents: 6 unit dose packs, 135 mg each. Product # 50841

Package Label – 270 mg
PROGRAM® (LUFENURON)
Once-a-month oral suspension
For the control of flea populations in cats
Recommended for cats 11 to 20 lbs. in weight.
Keep this and all drugs out of the reach of children.
Manufactured for: Novartis Animal Health US, Inc. Greensboro, NC 27408 U.S.A.
NADA # 141-026 Approved by the FDA www.petwellness.com
GIVE WITH A MEAL Net contents: 6 unit dose packs, 270 mg each. Product # 50845

ProgramLufenuron SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ProgramLufenuron SUSPENSION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||