Proforce Commercial Products
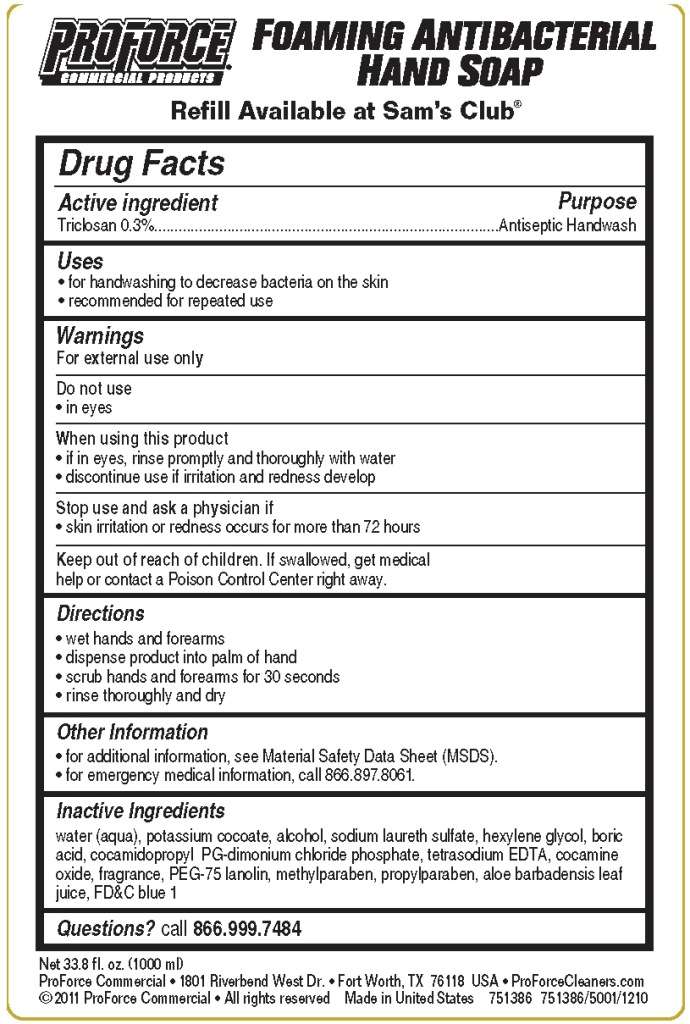
Drug Facts
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Proforce Commercial Products Uses
- Warnings
- Directions
- Proforce Commercial Products Other information
- Inactive Ingredients
- Principal display panel and representative label
FULL PRESCRIBING INFORMATION
Active ingredient
Triclosan 0.3%
Purpose
Antiseptic Handwash
Proforce Commercial Products Uses
- for handwashing to decrease bacteria on the skin
- recommended for repeated use
Warnings
For external use only
Do not use
- in eyes
When using this product
- if in eyes, rinse promptly and thoroughly with water
- discontinue use if irritation and redness develop
Stop use and ask a physician if
- skin irritation and redness occurs for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- wet hand and forearms
- dispense product into palm of hand
- scrub hands and forearms fro 30 seconds
- rinse thoroughly and dry
Proforce Commercial Products Other information
- for additional information, see Material Safety Data Sheet (MSDS).
- for emergency medical information, call 866.897.8061
Inactive Ingredients
water (aqua), potassium cocoate, alcohol, sodium laureth sulfate, hexylene glycol, boric acid, cocamidopropyl PG-dimonium chloride phosphate, tetrasodium EDTA, cocamine oxide, fragrance, PEG-75 lanolin, methylparaben, propylparaben, aloe barbadensis leaf juice, FDC blue 1
Questions? call 866.999.7484
Principal display panel and representative label
PROFORCE COMMERCIAL PRODUCTS
FOAMING ANTIBACTERIAL HAND SOAP
Refill Available at Sam's Club, copyright
Net 33.8 fl. oz. (1000 ml)
ProForce Commerical, 1801 Riverbend West Dr., Fort Worth, TX 76118 USA, ProForceCleaners.com
copyright 2011 ProForce Commercial, All rights reserved Made in United States 751386 751386/5001/1210
Proforce Commercial Productstriclosan SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||