Primaquine Phosphate
FULL PRESCRIBING INFORMATION
PHYSICIANS SHOULD COMPLETELY FAMILIARIZE THEMSELVES WITH THE COMPLETE CONTENTS OF THIS LEAFLET BEFORE PRESCRIBING PRIMAQUINE PHOSPHATE.
Primaquine phosphate is 8-[(4-Amino-1-methylbutyl)amino]-6-methoxyquinoline phosphate, a synthetic compound with potent antimalarial activity. Each tablet contains 26.3 mg of Primaquine phosphate (equivalent to 15 mg of primaquine base). The dosage is customarily expressed in terms of the base.
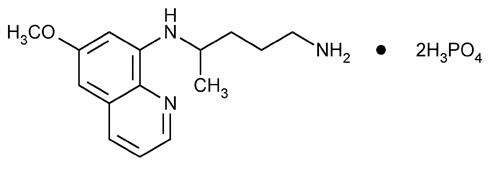
Molecular Formula C15H21N3O·2H3PO4 Molecular Wt 455.34
Inactive Ingredients: Colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, pregelatinized starch, talc, titanium dioxide, FD&C Yellow #5 aluminum lake, FD&C Yellow #6 aluminum lake, FD&C Blue #2 aluminum lake and FD&C Red #40 aluminum Lake.
Primaquine phosphate is an 8-aminoquinoline compound which eliminates tissue (exoerythrocytic) infection. Thereby, it prevents the development of the blood (erythrocytic) forms of the parasite which are responsible for relapses in vivax malaria. Primaquine phosphate is also active against gametocytes of Plasmodium falciparum.
Primaquine phosphate is indicated for the radical cure (prevention of relapse) of vivax malaria.
Primaquine phosphate is contraindicated in acutely ill patients suffering from systemic disease manifested by tendency to granulocytopenia, such as rheumatoid arthritis and lupus erythematosus. The drug is also contraindicated in patients receiving concurrently other potentially hemolytic drugs or depressants of myeloid elements of the bone marrow.
Because quinacrine hydrochloride appears to potentiate the toxicity of antimalarial compounds which are structurally related to primaquine, the use of quinacrine in patients receiving primaquine is contraindicated. Similarly, Primaquine should not be administered to patients who have received quinacrine recently, as toxicity is increased.
Discontinue the use of Primaquine phosphate promptly if signs suggestive of hemolytic anemia occur (darkening of the urine, marked fall of hemoglobin or erythrocytic count).
Hemolytic reactions (moderate to severe) may occur in individuals with glucose-6-phosphate dehydrogenase (G-6-PD) deficiency and in individuals with a family or personal history of favism. Areas of high prevalence of G-6-PD deficiency are Africa, Southern Europe, Mediterranean region, Middle East, South-East Asia, and Oceania. People from these regions have a greater tendency to develop hemolytic anemia (due to a congenital deficiency of erythrocytic glucose-6-phosphate dehydrogenase) while receiving Primaquine and related drugs.
Safe usage of this preparation in pregnancy has not been established. Therefore, use of it during pregnancy should be avoided except when in the judgment of the physician the benefit outweighs the possible hazard.
Since anemia, methemoglobinemia, and leukopenia have been observed following administration of large doses of primaquine, the adult dosage of 1 tablet (= 15 mg base) daily for fourteen days should not be exceeded. It is also advisable to make routine blood examinations (particularly blood cell counts and hemoglobin determinations) during therapy.
If primaquine phosphate is prescribed for (1) an individual who has shown a previous idiosyncrasy to primaquine phosphate (as manifested by hemolytic anemia, methemoglobinemia, or leukopenia), (2) an individual with a family or personal history of favism, or (3) an individual with erythrocytic glucose-6-phosphate dehydrogenase (G-6-PD) deficiency or nicotinamide adenine dinucleotide (NADH) methemoglobin reductase deficiency, the person should be observed closely for tolerance. The drug should be discontinued immediately if marked darkening of the urine or sudden decrease in hemoglobin concentration or leukocyte count occurs.
Clinical studies of primaquine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Gastrointestinal: nausea, vomiting, epigastric distress, and abdominal cramps.
Hematologic: leukopenia, hemolytic anemia in glucose-6-phosphate dehydrogenase (G-6-PD) deficient individuals, and methemoglobinemia in nicotinamide adenine dinucleotide (NADH) methemoglobin reductase deficient individuals.
Symptoms of overdosage of primaquine phosphate are similar to those seen after overdosage of pamaquine. They include abdominal cramps, vomiting, burning epigastric distress, central nervous system and cardiovascular disturbances, cyanosis, methemoglobinemia, moderate leukocytosis or leukopenia, and anemia. The most striking symptoms are granulocytopenia and acute hemolytic anemia in sensitive persons. Acute hemolysis occurs, but patients recover completely if the dosage is discontinued.
Primaquine phosphate is recommended only for the radical cure of vivax malaria, the prevention of relapse in vivax malaria, or following the termination of chloroquine phosphate suppressive therapy in an area where vivax malaria is endemic. Patients suffering from an attack of vivax malaria or having parasitized red blood cells should receive a course of chloroquine phosphate, which quickly destroys the erythrocytic parasites and terminates the paroxysm. Primaquine phosphate should be administered concurrently in order to eradicate the exoerythrocytic parasites in a dosage of 1 tablet (equivalent to 15 mg base) daily for 14 days.
Primaquine Phosphate Tablets USP contain primaquine phosphate 26.3 mg, equivalent to 15 mg base with "ALV" over "331" printed in black ink on one side and blank on the other, supplied as brown, round, film-coated tablets.
Available in bottles of 14 (NDC 47781-331-14)
Available in bottles of 30 (NDC 47781-331-30)
Available in bottles of 1000 (NDC 47781-331-10)
Store at 25° C (77° F); excursions permitted to 15° – 30° C (59° – 86° F) [see USP Controlled Room Temperature]
Dispense in tight, light-resistant container as defined in the USP/NF.
Malariologists agree that malaria produced by Plasmodium vivax is the most difficult form to treat. This is ascribed to the ability of the parasite to develop extremely resistant tissue forms which are not eradicated by ordinary antimalarial compounds.
Thus, persons with acute attacks of vivax malaria, provoked by the release of erythrocytic forms of the parasite, respond readily to therapy, particularly to Chloroquine phosphate. However, prior to the discovery of primaquine phosphate, no antimalarial drug was available that could be relied on to eliminate tissue (exoerythrocytic) infection and to prevent relapses. The various investigations made with primaquine in experimentally induced vivax malaria in human volunteers and in persons with naturally occurring infections have demonstrated that the drug is a valuable adjunct to conventional therapy in this refractory form of the disease.
Made in USA
Dist by:
Alvogen, Inc.
Pine Brook, NJ 07058 USA
Rev: 04/2012
PI331-01



Primaquine PhosphatePrimaquine Phosphate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||