PreferaOB
PreferaOB Prenatal/Postnatal Multivitamin/ Multimineral Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- PREFERAOB DESCRIPTION
- PREFERAOB INDICATIONS AND USAGE
- PREFERAOB CONTRAINDICATIONS
- WARNING
- PRECAUTIONS
- PREFERAOB ADVERSE REACTIONS
- PREFERAOB DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
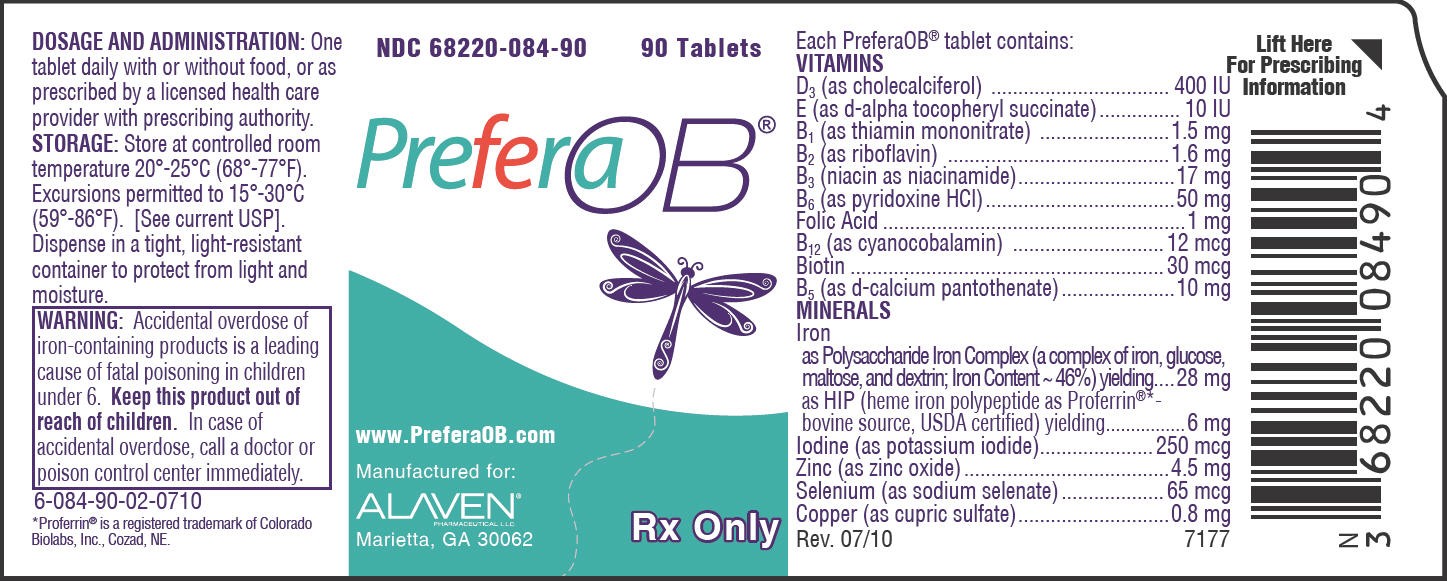
- PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
FULL PRESCRIBING INFORMATION
Rx Only
PREFERAOB DESCRIPTION
PreferaOB® is a prescription prenatal/postnatal multivitamin/multimineral nutritional supplement. PreferaOB® is a small, oval, film-coated, purple tablet with black core, debossed and bisected with "AP/84" on one side, and plain on the other.
|
Each PreferaOB® tablet contains: VITAMINS |
|
| D3 (as cholecalciferol) | 400 IU |
| E (as d-alpha tocopheryl succinate) | 10 IU |
| B1 (as thiamin mononitrate) | 1.5 mg |
| B2 (as riboflavin) | 1.6 mg |
| B3 (niacin as niacinamide) | 17 mg |
| B6 (as pyridoxine HCl) | 50 mg |
| Folic Acid | 1 mg |
| B12 (as cyanocobalamin) | 12 mcg |
| Biotin | 30 mcg |
| B5 (as d-calcium pantothenate) | 10 mg |
| MINERALS | |
| Iron | |
| as Polysaccharide Iron Complex (a complex of iron, glucose, maltose, and dextrin; Iron Content ~ 46%) yielding | 28 mg |
| as HIP (heme iron polypeptide as Proferrin®*- bovine source, USDA certified) yielding | 6 mg |
| Iodine (as potassium iodide) | 250 mcg |
| Zinc (as zinc oxide) | 4.5 mg |
| Selenium (as sodium selenate) | 65 mcg |
| Copper (as cupric sulfate) | 0.8 mg |
INACTIVE INGREDIENTS: Microcrystalline cellulose, croscarmellose sodium, crospovidone, magnesium stearate, silicon dioxide, dextran, dicalcium phosphate, sucrose, protease, modified food starch, dl-alpha tocopherol, sodium ascorbate, ethylcellulose, resin, coating (polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, D&C red no. 30 aluminum lake, FD&C blue no. 2 aluminum lake).
PREFERAOB INDICATIONS AND USAGE
PreferaOB® is a prescription multivitamin/multimineral nutritional supplement indicated for use in improving the nutritional status of women throughout pregnancy and in the postnatal period for both lactating and non-lactating mothers. PreferaOB® is also beneficial in improving the nutritional status of women prior to conception.
PREFERAOB CONTRAINDICATIONS
This product is contraindicated in patients with a known hypersensitivity to any of the ingredients.
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
PRECAUTIONS
Folic acid when administered as a single agent in doses above 0.1 mg daily may obscure pernicious anemia in that hematological remission can occur while neurological manifestations remain progressive. Pregnant women and nursing mothers should avoid supplemental doses of vitamin E higher than RDA amounts. While prescribing this nutritional supplement for pregnant women, nursing mothers, or for women prior to conception, their medical condition and other drugs, herbs, and/or supplements consumption should be considered.
PREFERAOB ADVERSE REACTIONS
Allergic sensitization has been reported following both oral and parenteral administration of folic acid.
PREFERAOB DOSAGE AND ADMINISTRATION
One tablet daily with or without food, or as prescribed by a licensed health care provider with prescribing authority.
HOW SUPPLIED
PreferaOB® is supplied in a child-resistant bottle of 90 tablets (NDC 68220-084-90) and as a Professional Sample (NDC 68220-084-25).
KEEP OUT OF REACH OF CHILDREN.
STORAGE
Store at controlled room temperature 20°-25°C (68°-77°F). Excursions permitted to 15°-30°C (59°-86°F). [See current USP]
Dispense in a tight, light-resistant container to protect from light and moisture.
For medical inquiries call toll free 1-888-317-0001
www.PreferaOB.com
Manufactured for:
ALAVEN
®
PHARMACEUTICAL LLC
Marietta, GA.
U.S. Patent No. 7,585,527
*Proferrin® is a registered trademark of Colorado Biolabs, Inc., Cozad, NE.
PreferaOB® is a registered trademark of Alaven Pharmaceutical LLC,
Marietta, GA 30062
6-084-90-03-0710CR
CL6-084-00-01-0710a
PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
NDC 68220-084-90
90 Tablets
PreferaOB ®
www.PreferaOB.com
Manufactured for:
ALAVEN
®
PHARMACEUTICAL LLC
Marietta, GA 30062
Rx Only
PreferaOBFOLIC ACID, Heme Iron Polypeptide, IRON DEXTRAN, Potassium Iodide, Zinc Oxide, Selenium, CUPRIC SULFATE, CHOLECALCIFEROL, THIAMINE MONONITRATE, ALPHA-TOCOPHEROL SUCCINATE, D-, RIBOFLAVIN, NIACINAMIDE, PYRIDOXINE HYDROCHLORIDE, CYANOCOBALAMIN, BIOTIN, and CALCIUM PANTOTHENATE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||