Pravastatin Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- PRAVASTATIN SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS/ METABOLISM
- CLINICAL STUDIES
- INDICATIONS & USAGE
- PRAVASTATIN SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- PRAVASTATIN SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
PRAVASTATIN SODIUM DESCRIPTION
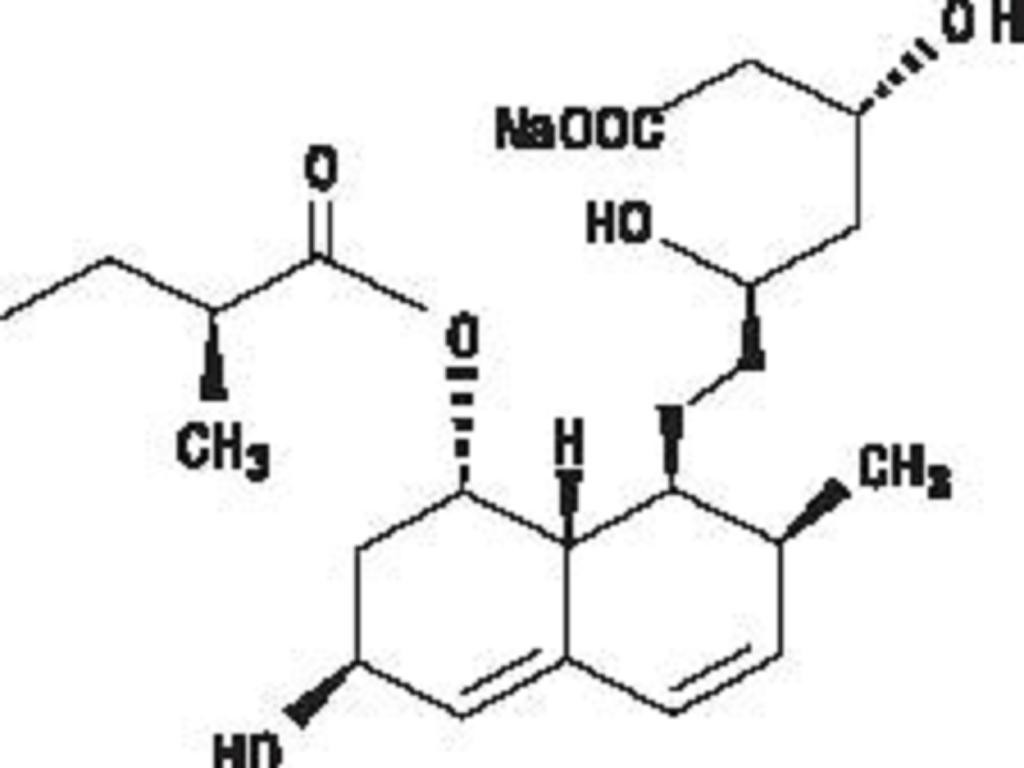
CLINICAL PHARMACOLOGY
Clinical Studies
PHARMACOKINETICS/ METABOLISM
CLINICAL STUDIES
Prevention of Coronary Heart Disease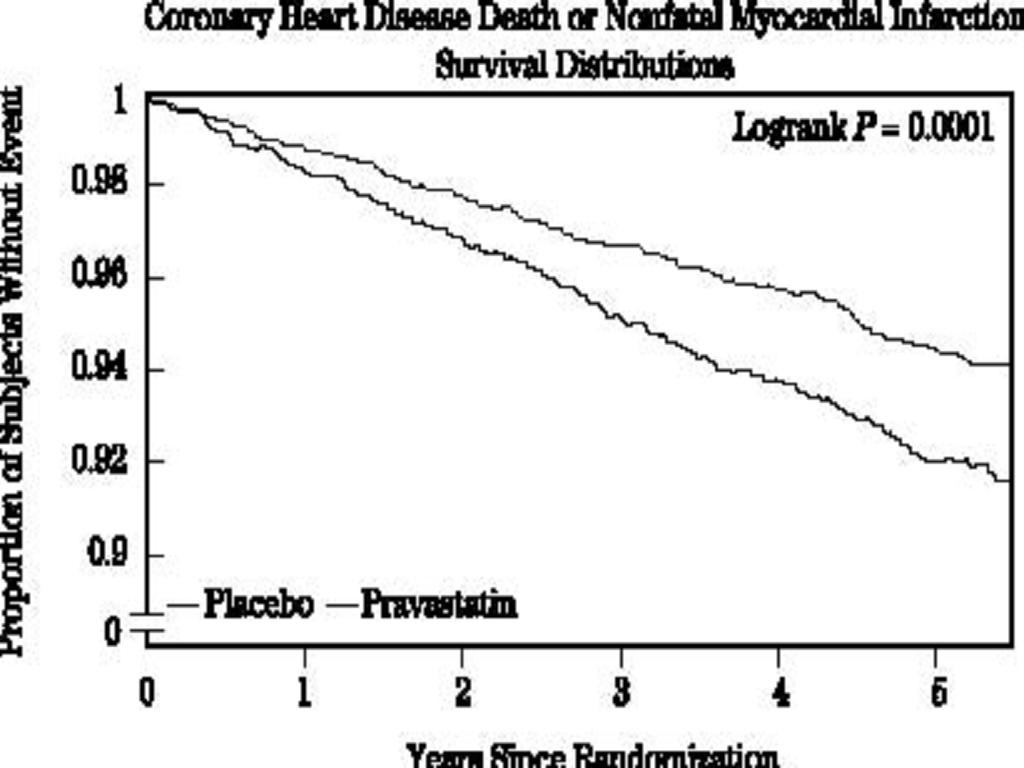
Secondary Prevention of Cardiovascular Events
Primary Hypercholesterolemia (Fredrickson Type IIa and IIb)
**
Hypertriglyceridemia (Fredrickson Type IV)
Dysbetalipoproteinemia (Fredrickson Type III)
Pediatric Clinical Study
*
*
INDICATIONS & USAGE
Primary Prevention of Coronary Events
-
● Reduce the risk of myocardial infarction
-
● Reduce the risk of undergoing myocardial revascularization procedures
-
● Reduce the risk of cardiovascular mortality with no increase in death from non-cardiovascular causes.
Hyperlipidemia
-
● there is a positive family history of premature cardiovascular disease or
-
● two or more other CVD risk factors are present in the patient.
*Risk CategoryLDL Goal (mg/dL)LDL Level at Which to Initiate Therapeutic Lifestyle Changes (mg/dL)LDL Levels at Which to Consider Drug Therapy (mg/dL)CHD*or CHD Risk equivalents (10-year risk >20%)<100(100-129: drug optional)2+ Risk factors (10-year risk<13010-year risk 10%-20%:10-year risk <10%:0-1 Risk factor<160(160-189: LDL-lowering drug optional)After the LDL-C goal has been achieved, if the TG is stillmg/dL, non-HDL-C (Total-C minus HDL-C) becomes a secondary target of therapy. Non-HDL-C goals are set 30 mg/dL higher than LDL-C goals for each risk category.
At the time of hospitalization for an acute coronary event, consideration can be given to initiating drug therapy at discharge if the LDL-C ismg/dL (see NCEP Treatment Guidelines, above).
Since the goal of treatment is to lower LDL-C, the NCEP recommends that LDL-C levels be used to initiate and assess treatment response. Only if LDL-C levels are not available, should the Total-C be used to monitor therapy.
As with other lipid-lowering therapy, pravastatin sodium tablets are not indicated when hypercholesterolemia is due to hyperalphalipoproteinemia (elevated HDL-C).
The NCEP classification of cholesterol levels in pediatric patients with a familial history of hypercholesterolemia or premature cardiovascular disease is summarized below:
CategoryTotal-C (mg/dL)LDL-C (mg/dL)Acceptable<170<110Borderline170-199110-129High
PRAVASTATIN SODIUM CONTRAINDICATIONS
WARNINGS
Pregnancy and Lactation.
PRECAUTIONS: Pregnancy
WARNINGS
Liver EnzymesCLINICAL PHARMACOLOGY: Clinical Studiesin both treatment groups. Overall, clinical trial experience showed that liver function test abnormalities observed during pravastatin therapy were usually asymptomatic, not associated with cholestasis, and did not appear to be related to treatment duration. In a 320-patient placebo-controlled clinical trial, subjects with chronic (>6 months) stable liver disease, due primarily to hepatitis C or non-alcoholic fatty liver disease, were treated with 80 mg pravastatin or placebo for up to 9 months. The primary safety endpoint was the proportion of subjects with at least one ALTtimes the upper limit of normal for those with normal ALT (the upper limit of normal) at baseline or a doubling of the baseline ALT for those with elevated ALT (> the upper limit of normal) at baseline. By Week 36, 12 out of 160 (7.5%) subjects treated with pravastatin met the prespecified safety ALT endpoint compared to 20 out of 160 (12.5%) subjects receiving placebo. Conclusions regarding liver safety are limited since the study was not large enough to establish similarity between groups (with 95% confidence) in the rates of ALT elevation.
It is recommended that liver function tests be performed prior to the initiation of therapy and when clinically indicated.
Active liver disease or unexplained persistent transaminase elevations are contraindications to the use of pravastatin (seeCONTRAINDICATIONS). Caution should be exercised when pravastatin is administered to patients who have a recent (< 6 months) history of liver disease, have signs that may suggest liver disease (e.g., unexplained aminotransferase elevations, jaundice), or are heavy users of alcohol (seeCLINICAL PHARMACOLOGY: Pharmacokinetics/Metabolism). Such patients should be closely monitored, started at the lower end of the recommended dosing range (seeDOSAGE AND ADMINISTRATION: Adult Patients), and titrated to the desired therapeutic effect.
Patients who develop increased transaminase levels or signs and symptoms of active liver disease while taking pravastatin should be evaluated with a second liver function evaluation to confirm the finding and be followed thereafter with frequent liver function tests until the abnormality(ies) return to normal. Should an increase in AST or ALT of three times the upper limit of normal or greater persist, withdrawal of pravastatin therapy is recommended.
Skeletal Muscle
Rare cases of rhabdomyolysis with acute renal failure secondary to myoglobinuria have been reported with pravastatin and other drugs in this class.Uncomplicated myalgia has also been reported in pravastatin-treated patients (seeADVERSE REACTIONS). Myopathy, defined as muscle aching or muscle weakness in conjunction with increases in creatine phosphokinase (CPK) values to greater than 10 times the upper limit of normal, was rare (<0.1%) in pravastatin clinical trials. Myopathy should be considered in any patient with diffuse myalgias, muscle tenderness or weakness, and/or marked elevation of CPK. Patients should be advised to report promptly unexplained muscle pain, tenderness or weakness, particularly if accompanied by malaise or fever. Pravastatin therapy should be discontinued if markedly elevated CPK levels occur or myopathy is diagnosed or suspected.
The risk of myopathy during treatment with another HMG-CoA reductase inhibitor is increased with concurrent therapy with either erythromycin, cyclosporine, niacin, or fibrates. However, neither myopathy nor significant increases in CPK levels have been observed in three reports involving a total of 100 post-transplant patients (24 renal and 76 cardiac) treated for up to two years concurrently with pravastatin 10-40 mg and cyclosporine. Some of these patients also received other concomitant immunosuppressive therapies. Further, in clinical trials involving small numbers of patients who were treated concurrently with pravastatin and niacin, there were no reports of myopathy. Also, myopathy was not reported in a trial of combination pravastatin (40 mg/day) and gemfibrozil (1200 mg/day), although 4 of 75 patients on the combination showed marked CPK elevations versus one of 73 patients receiving placebo. There was a trend toward more frequent CPK elevations and patient withdrawals due to musculoskeletal symptoms in the group receiving combined treatment as compared with the groups receiving placebo, gemfibrozil, or pravastatin monotherapy (seePRECAUTIONS: Drug interactions).The use of fibrates alone may occasionally be associated with myopathy. The combined use of pravastatin and fibrates should be avoided unless the benefit of further alterations in lipid levels is likely to outweigh the increased risk of this drug combination.
PRECAUTIONS
GeneralADVERSE REACTIONS
Homozygous Familial Hypercholesterolemia.
Renal Insufficiency.
INFORMATION FOR PATIENTS
WARNINGS: Skeletal MuscleDRUG INTERACTIONS
Immunosuppressive Drugs, Gemfibrozil, Niacin (Nicotinic Acid), Erythromycin:WARNINGS: Skeletal MuscleCytochrome P450 3A4 Inhibitors
Diltiazem:
Itraconazole:
Antipyrine:
Cholestyramine/Colestipol:DOSAGE AND ADMINISTRATION: Concomitant Therapy
Warfarin:
Cimetidine
Digoxin:
Cyclosporine:
Gemfibrozil:WARNINGS: Skeletal Muscle
Endocrine Function
CNS Toxicity
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Pregnancy Category X.CONTRAINDICATIONS
CONTRAINDICATIONS
NURSING MOTHERS
CONTRAINDICATIONSPEDIATRIC USE
ADVERSE REACTIONS: Pediatric PatientsDoses greater than 40 mg have not been studied in this population.CONTRAINDICATIONSPregnancyDOSAGE AND ADMINISTRATION: Adult PatientsPediatric PatientsGERIATRIC USE
CLINICAL PHARMACOLOGY: Pharmacokinetics/Metabolism
PRAVASTATIN SODIUM ADVERSE REACTIONS
PRECAUTIONS: Geriatric useAdverse Clinical Events
Short-Term Controlled Trials
**
Long-Term Controlled Morbidity and Mortality Trials
Dermatologic:
Endocrine/Metabolic
Gastrointestinal:
General:
Immunologic:
Musculoskeletal:
Nervous System
Special Senses:
Postmarketing Experience
Musculoskeletal:
Nervous System:
Hypersensitivity:
Gastrointestinal
Dermatologic:
Reproductive
Laboratory Abnormalities:
Laboratory Test Abnormalities
WARNINGS
Concomitant Therapy
WARNINGS: Skeletal MusclePRECAUTIONS: Drug interactions
Pediatric Patients
CLINICAL PHARMACOLOGY: Pediatric Clinical StudyPRECAUTIONS: Pediatric use
OVERDOSAGE
WARNINGSDOSAGE & ADMINISTRATION
Adult Patients
Pediatric Patients
Children (Ages 8 to 13 Years, Inclusive)
Adolescents (Ages 14 to 18 Years)
INDICATIONS AND USAGE: Hyperlipidemia, NCEP Treatment Guidelines
WARNINGS: Skeletal Muscle
Concomitant Therapy
ADVERSE REACTIONS: Concomitant Therapy
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Pravastatin SodiumPravastatin Sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!