PIOGLITAZONEHYDROCHLORIDE
Torrent Pharmaceuticals Limited
Torrent Pharma, Inc.
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use pioglitazone hydrochloride safely and effectively. See full prescribing information for pioglitazone hydrochloride. Pioglitazone Tablets, USP for oral use Initial U.S. Approval: 1999 BOXED WARNINGWARNING: CONGESTIVE HEART FAILURESee full prescribing information for complete boxed warning. Thiazolidinediones, including pioglitazone hydrochloride, cause or exacerbate congestive heart failure in some patients. (5.1) After initiation of pioglitazone hydrochloride, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride must be considered. (5.1) Pioglitazone hydrochloride is not recommended in patients with symptomatic heart failure. (5.1) Initiation of pioglitazone hydrochloride in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. (4, 5.1) INDICATIONS AND USAGEPioglitazone tablets, USP are a thiazolidinedione and an agonist for peroxisome proliferator-activated receptor (PPAR) gamma indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings. (1, 14) Important Limitation of Use: Not for treatment of type 1 diabetes or diabetic ketoacidosis. (1) DOSAGE AND ADMINISTRATION Initiate pioglitazone tablets at 15 mg or 30 mg once daily. Limit initial dose to 15 mg once daily in patients with NYHA Class I or II heart failure. (2.1) If there is inadequate glycemic control, the dose can be increased in 15 mg increments up to a maximum of 45 mg once daily. (2.1) Obtain liver tests before starting pioglitazone tablets. If abnormal, use caution when treating with pioglitazone tablets, investigate the probable cause, treat (if possible) and follow appropriately. Monitoring liver tests while on pioglitazone tablets is not recommended in patients without liver disease. (5.3) DOSAGE FORMS AND STRENGTHSTablets: 15 mg, 30 mg, and 45 mg (3)CONTRAINDICATIONS Initiation in patients with established New York Heart Association (NYHA) Class III or IV heart failure [see Boxed Warning] . (4) Use in patients with known hypersensitivity to pioglitazone or any other component of pioglitazone hydrochloride. (4) WARNINGS AND PRECAUTIONS Congestive heart failure: Fluid retention may occur and can exacerbate or lead to congestive heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk. Monitor patients for signs and symptoms. (5.1) Hypoglycemia: When used with insulin or an insulin secretagogue, a lower dose of the insulin or insulin secretagogue may be needed to reduce the risk of hypoglycemia. (5.2) Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt pioglitazone hydrochloride and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart pioglitazone hydrochloride if liver injury is confirmed and no alternate etiology can be found. (5.3) Bladder cancer: Preclinical and clinical trial data, and results from an observational study suggest an increased risk of bladder cancer in pioglitazone users. The observational data further suggest that the risk increases with duration of use. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer (5.4) Edema: Dose-related edema may occur. (5.5) Fractures: Increased incidence in female patients. Apply current standards of care for assessing and maintaining bone health. (5.6) Macular edema: Postmarketing reports. Recommend regular eye exams in all patients with diabetes according to current standards of care with prompt evaluation for acute visual changes. (5.7) Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone hydrochloride or any other antidiabetic drug. (5.9) Side EffectsMost common adverse reactions (≥5%) are upper respiratory tract infection, headache, sinusitis, myalgia, and pharyngitis. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Torrent Pharma Inc. at 1-269-544-2299 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. DRUG INTERACTIONS Strong CYP2C8 inhibitors (e.g., gemfibrozil) increase pioglitazone concentrations. Limit pioglitazone hydrochloride dose to 15 mg daily. (2.3, 7.1) CYP2C8 inducers (e.g., rifampin) may decrease pioglitazone concentrations. (7.2) USE IN SPECIFIC POPULATIONS Nursing mothers: Discontinue drug or nursing, taking into consideration the importance of the drug to the mother (8.3) Pediatrics: Not recommended for use in pediatric patients. (8.4)
FULL PRESCRIBING INFORMATION: CONTENTS*
- WARNING: CONGESTIVE HEART FAILURE
- 1. PIOGLITAZONEHYDROCHLORIDE INDICATIONS AND USAGE
- 2. PIOGLITAZONEHYDROCHLORIDE DOSAGE AND ADMINISTRATION
- 3. DOSAGE FORMS AND STRENGTHS
- 4. PIOGLITAZONEHYDROCHLORIDE CONTRAINDICATIONS
- 5. WARNINGS AND PRECAUTIONS
- 6. PIOGLITAZONEHYDROCHLORIDE ADVERSE REACTIONS
- 7. DRUG INTERACTIONS
- 8. USE IN SPECIFIC POPULATIONS
- 10. OVERDOSAGE
- 11. PIOGLITAZONEHYDROCHLORIDE DESCRIPTION
- 12. CLINICAL PHARMACOLOGY
- 13. NONCLINICAL TOXICOLOGY
- 14. CLINICAL STUDIES
- 16. HOW SUPPLIED/STORAGE AND HANDLING
- 17. PATIENT COUNSELING INFORMATION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
WARNING: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including pioglitazone hydrochloride, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.1)] .
- After initiation of pioglitazone hydrochloride, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride must be considered.
- Pioglitazone hydrochloride is not recommended in patients with symptomatic heart failure.
- Initiation of pioglitazone hydrochloride in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)] .
1. INDICATIONS AND USAGE
Monotherapy and Combination Therapy
Pioglitazone tablets, USP are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus in multiple clinical settings [see Clinical Studies (14)].
Important Limitation of Use
Pioglitazone tablets, USP exert its antihyperglycemic effect only in the presence of endogenous insulin. Pioglitazone tablets, USP should not be used to treat type 1 diabetes or diabetic ketoacidosis, as it would not be effective in these settings.
Use caution in patients with liver disease [see Warnings and Precautions (5.3)].
2. DOSAGE AND ADMINISTRATION
2.1 Recommendations for All Patients
Pioglitazone tablets should be taken once daily and can be taken without regard to meals.
The recommended starting dose for patients without congestive heart failure is 15 mg or 30 mg once daily.
The recommended starting dose for patients with congestive heart failure (NYHA Class I or II) is 15 mg once daily.
The dose can be titrated in increments of 15 mg up to a maximum of 45 mg once daily based on glycemic response as determined by HbA1c.
After initiation of pioglitazone tablets or with dose increase, monitor patients carefully for adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure [see Boxed Warning and Warnings and Precautions (5.5)].
Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating pioglitazone tablets. Routine periodic monitoring of liver tests during treatment with pioglitazone tablets is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of pioglitazone tablets or who are found to have abnormal liver tests while taking pioglitazone tablets should be managed as described under Warnings and Precautions [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
2.2 Concomitant Use with an Insulin Secretagogue or Insulin
If hypoglycemia occurs in a patient co-administered pioglitazone tablets and an insulin secretagogue (e.g., sulfonylurea), the dose of the insulin secretagogue should be reduced.
If hypoglycemia occurs in a patient co-administered pioglitazone tablets and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
2.3 Concomitant Use with Strong CYP2C8 Inhibitors
Coadministration of pioglitazone tablets and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3-fold. Therefore, the maximum recommended dose of pioglitazone tablets is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. DOSAGE FORMS AND STRENGTHS
Round tablet contains pioglitazone as follows:
- 15 mg: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '140' on one side and '15' on other side
- 30 mg: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '1119' on one side and plain on other side
- 45 mg: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '1120' on one side and plain on other side
4. CONTRAINDICATIONS
- Initiation in patients with established NYHA Class III or IV heart failure [see Boxed Warning] .
- Use in patients with known hypersensitivity to pioglitazone or any other component of pioglitazone hydrochloride.
5. WARNINGS AND PRECAUTIONS
5.1 Congestive Heart Failure
Pioglitazone hydrochloride, like other thiazolidinediones, can cause dose-related fluid retention when used alone or in combination with other antidiabetic medications and is most common when pioglitazone hydrochloride is used in combination with insulin. Fluid retention may lead to or exacerbate congestive heart failure. Patients should be observed for signs and symptoms of congestive heart failure. If congestive heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone hydrochloride must be considered [see Boxed Warning, Contraindications (4), and Adverse Reactions (6.1)].
5.2 Hypoglycemia
Patients receiving pioglitazone hydrochloride in combination with insulin or other antidiabetic medications (particularly insulin secretagogues such as sulfonylureas) may be at risk for hypoglycemia. A reduction in the dose of the concomitant antidiabetic medication may be necessary to reduce the risk of hypoglycemia [see Dosage and Administration (2.2)].
5.3 Hepatic Effects
There have been postmarketing reports of fatal and non-fatal hepatic failure in patients taking pioglitazone hydrochloride, although the reports contain insufficient information necessary to establish the probable cause. There has been no evidence of drug-induced hepatotoxicity in the pioglitazone hydrochloride controlled clinical trial database to date [see Adverse Reactions (6.1)].
Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (serum alanine aminotransferase [ALT], aspartate aminotransferase [AST], alkaline phosphatase, and total bilirubin) and assessing the patient is recommended before initiating pioglitazone hydrochloride therapy. In patients with abnormal liver tests, pioglitazone hydrochloride should be initiated with caution.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than 3 times the upper limit of the reference range), pioglitazone hydrochloride treatment should be interrupted and investigation done to establish the probable cause. Pioglitazone hydrochloride should not be restarted in these patients without another explanation for the liver test abnormalities.
Patients who have serum ALT greater than three times the reference range with serum total bilirubin greater than two times the reference range without alternative etiologies are at risk for severe drug-induced liver injury, and should not be restarted on pioglitazone hydrochloride. For patients with lesser elevations of serum ALT or bilirubin and with an alternate probable cause, treatment with pioglitazone hydrochloride can be used with caution.
5.4 Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In two 3-year trials in which pioglitazone hydrochloride was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone hydrochloride compared to 5/3679 (0.14%) in patients not taking pioglitazone hydrochloride. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone hydrochloride and two (0.05%) cases on placebo.
A five-year interim report of an ongoing 10-year observational cohort study found a non significant increase in the risk for bladder cancer in subjects ever exposed to pioglitazone hydrochloride, compared to subjects never exposed to pioglitazone hydrochloride (HR 1.2 [95% CI 0.9 – 1.5]). Compared to never exposure, a duration of pioglitazone hydrochloride therapy longer than 12 months was associated with an increase in risk (HR 1.4 [95% CI 0.9 – 2.1]), which reached statistical significance after more than 24 months of pioglitazone hydrochloride use (HR 1.4 [95% CI 1.03 – 2.0]). Interim results from this study suggested that taking pioglitazone hydrochloride longer than 12 months increased the relative risk of developing bladder cancer in any given year by 40% which equates to an absolute increase of three cases in 10,000 (from approximately seven in 10,000 [without pioglitazone hydrochloride] to approximately 10 in 10,000 [with pioglitazone hydrochloride]).
There are insufficient data to determine whether pioglitazone is a tumor promoter for urinary bladder tumors. Consequently, pioglitazone hydrochloride should not be used in patients with active bladder cancer and the benefits of glycemic control versus unknown risks for cancer recurrence with pioglitazone hydrochloride should be considered in patients with a prior history of bladder cancer.
5.5 Edema
In controlled clinical trials, edema was reported more frequently in patients treated with pioglitazone hydrochloride than in placebo-treated patients and is dose-related [see Adverse Reactions (6.1)]. In postmarketing experience, reports of new onset or worsening edema have been received.
Pioglitazone hydrochloride should be used with caution in patients with edema. Because thiazolidinediones, including pioglitazone hydrochloride, can cause fluid retention, which can exacerbate or lead to congestive heart failure, pioglitazone hydrochloride should be used with caution in patients at risk for congestive heart failure. Patients treated with pioglitazone hydrochloride should be monitored for signs and symptoms of congestive heart failure [see Boxed Warning, Warnings and Precautions (5.1) and Patient Counseling Information (17)].
5.6 Fractures
In PROactive (the Prospective Pioglitazone Clinical Trial in Macrovascular Events), 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone hydrochloride (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. During a mean follow-up of 34.5 months, the incidence of bone fracture in females was 5.1% (44/870) for pioglitazone hydrochloride versus 2.5% (23/905) for placebo. This difference was noted after the first year of treatment and persisted during the course of the study. The majority of fractures observed in female patients were nonvertebral fractures including lower limb and distal upper limb. No increase in the incidence of fracture was observed in men treated with pioglitazone hydrochloride (1.7%) versus placebo (2.1%). The risk of fracture should be considered in the care of patients, especially female patients, treated with pioglitazone hydrochloride and attention should be given to assessing and maintaining bone health according to current standards of care.
5.7 Macular Edema
Macular edema has been reported in postmarketing experience in diabetic patients who were taking pioglitazone hydrochloride or another thiazolidinedione. Some patients presented with blurred vision or decreased visual acuity, but others were diagnosed on routine ophthalmologic examination.
Most patients had peripheral edema at the time macular edema was diagnosed. Some patients had improvement in their macular edema after discontinuation of the thiazolidinedione.
Patients with diabetes should have regular eye exams by an ophthalmologist according to current standards of care. Patients with diabetes who report any visual symptoms should be promptly referred to an ophthalmologist, regardless of the patient's underlying medications or other physical findings [see Adverse Reactions (6.1)].
5.8 Ovulation
Therapy with pioglitazone hydrochloride, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking pioglitazone hydrochloride [see Use in Specific Populations (8.1)]. This effect has not been investigated in clinical trials, so the frequency of this occurrence is not known. Adequate contraception in all premenopausal women treated with pioglitazone hydrochloride is recommended.
5.9 Macrovascular Outcomes
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone hydrochloride or any other antidiabetic drug.
6. ADVERSE REACTIONS
The following serious adverse reactions are discussed elsewhere in the labeling:
- Congestive heart failure [see Boxed Warning and Warnings and Precautions (5.1)]
- Edema [see Warnings and Precautions (5.5)]
- Fractures [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Over 8500 patients with type 2 diabetes have been treated with pioglitazone hydrochloride in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone hydrochloride in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone hydrochloride for six months or longer, over 4500 patients have been treated with pioglitazone hydrochloride for one year or longer, and over 3000 patients have been treated with pioglitazone hydrochloride for at least two years.
In six pooled 16- to 26-week placebo-controlled monotherapy and 16- to 24-week add-on combination therapy trials, the incidence of withdrawals due to adverse events was 4.5% for patients treated with pioglitazone hydrochloride and 5.8% for comparator-treated patients. The most common adverse events leading to withdrawal were related to inadequate glycemic control, although the incidence of these events was lower (1.5%) with pioglitazone hydrochloride than with placebo (3.0%).
In the PROactive trial, the incidence of withdrawals due to adverse events was 9.0% for patients treated with pioglitazone hydrochloride and 7.7% for placebo-treated patients. Congestive heart failure was the most common serious adverse event leading to withdrawal occurring in 1.3% of patients treated with pioglitazone hydrochloride and 0.6% of patients treated with placebo.
Common Adverse Events: 16- to 26-Week Monotherapy Trials
A summary of the incidence and type of common adverse events reported in three pooled 16- to 26-week placebo-controlled monotherapy trials of pioglitazone hydrochloride is provided in Table 1. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone hydrochloride than in patients who received placebo. None of these adverse events were related to pioglitazone hydrochloride dose.
|
Table
1
.
Three
Pooled
16
-
to
26
-
Week
Placebo
-
Controlled
Clinical
Trials
of
Pioglitazone
Hydrochloride
Monotherapy
:
Adverse
Events
Reported
at
an
Incidence
>
5
%
and
More
Commonly in Patients Treated with Pioglitazone Hydrochloride than in Patients Treated with Placebo |
||
|
(%
of
Patients
)
|
||
|
|
Placebo
N=259 |
Pioglitazone
Hydrochloride
N=606 |
| Upper Respiratory Tract Infection
|
8.5
|
13.2
|
| Headache
|
6.9
|
9.1
|
| Sinusitis
|
4.6
|
6.3
|
| Myalgia
|
2.7
|
5.4
|
| Pharyngitis
|
0.8
|
5.1
|
Common Adverse Events: 16- to 24-Week Add-on Combination Therapy Trials
A summary of the overall incidence and types of common adverse events reported in trials of pioglitazone hydrochloride add-on to sulfonylurea is provided in Table 2. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone hydrochloride.
|
Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.” |
|||
|
Table
2
.
16
-
to
24
-
Week
Clinical
Trials
of
Pioglitazone
Hydrochloride
Add
-
on
to
Sulfonylurea
|
|||
|
|
16
-
Week
Placebo
-
Controlled
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated
with
Pioglitazone Hydrochloride 30 mg + Sulfonylurea than in Patients Treated with Placebo + Sulfonylurea |
||
|
|
%
of
Patients
|
||
|
|
Placebo
+
Sulfonylurea
N=187 |
Pioglitazone
Hydrochloride
15
mg
+
Sulfonylurea N=184 |
Pioglitazone
Hydrochloride
30
mg
+
Sulfonylurea N=189 |
| Edema |
2.1 |
1.6 |
12.7 |
| Headache |
3.7 |
4.3 |
5.3 |
| Flatulence |
0.5 |
2.7 |
6.3 |
| Weight Increased |
0 |
2.7 |
5.3 |
|
|
24
-
Week
Non
-
Controlled
Double
-
Blind
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated
with Pioglitazone Hydrochloride 45 mg + Sulfonylurea than in Patients Treated with Pioglitazone Hydrochloride 30 mg + Sulfonylurea |
||
|
|
%
of
Patients
|
||
|
|
PioglitazoneHydrochloride
30
mg
+
Sulfonylurea
N=351 |
Pioglitazone
Hydrochloride
45
mg
+
Sulfonylurea
N=351 |
|
| Hypoglycemia |
13.4 |
15.7 |
|
| Edema |
10.5 |
23.1 |
|
| Upper Respiratory Tract Infection |
12.3 |
14.8 |
|
| Weight Increased |
9.1 |
13.4 |
|
| Urinary Tract Infection |
5.7 |
6.8 |
|
A summary of the overall incidence and types of common adverse events reported in trials of pioglitazone hydrochloride add-on to metformin is provided in Table 3. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone hydrochloride.
|
Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.” |
||
|
Table
3
.
16
-
to
24
-
Week
Clinical
Trials
of
Pioglitazone
Hydrochloride
Add
-
on
to
Metformin
|
||
|
|
16
-
Week
Placebo
-
Controlled
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated
with Pioglitazone Hydrochloride + Metformin than in Patients Treated with Placebo + Metformin |
|
|
|
%
of
Patients
|
|
|
|
Placebo
+
Metformin
N=160 |
Pioglitazone
Hydrochloride30
mg
+
Metformin
N=168 |
| Edema |
2.5 |
6.0 |
| Headache |
1.9 |
6.0 |
|
|
24
-
Week
Non
-
Controlled
Double
-
Blind
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated with Pioglitazone Hydrochloride 45 mg + Metformin than in Patients Treated with Pioglitazone Hydrochloride 30 mg + Metformin |
|
|
|
%
of
Patients
|
|
|
|
Pioglitazone
Hydrochloride
30
mg
+
Metformin
N = 411 |
Pioglitazone
Hydrochloride
45
mg
+
Metformin
N = 416 |
| Upper Respiratory Tract Infection |
12.4 |
13.5 |
| Edema |
5.8 |
13.9 |
| Headache |
5.4 |
5.8 |
| Weight Increased |
2.9 |
6.7 |
Table 4 summarizes the incidence and types of common adverse events reported in trials of pioglitazone hydrochloride add-on to insulin. Terms that are reported represent those that occurred at an incidence of >5% and more commonly with the highest tested dose of pioglitazone hydrochloride.
|
Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.” |
|||
|
Table
4
.
16
-
to
24
-
Week
Clinical
Trials
of
Pioglitazone
Hydrochloride
Add
-
on
to
Insulin
|
|||
|
|
16
-
Week
Placebo
-
Controlled
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated
with Pioglitazone Hydrochloride 30 mg + Insulin than in Patients Treated with Placebo + Insulin |
||
|
|
%
of
Patients
|
||
|
|
Placebo
+
Insulin
N=187 |
Pioglitazone
Hydrochloride
15
mg
+ Insulin N = 191 |
Pioglitazone
Hydrochloride
30
mg
+ Insulin N = 188 |
| Hypoglycemia |
4.8 |
7.9 |
15.4 |
| Edema |
7.0 |
12.6 |
17.6 |
| Upper Respiratory Tract Infection |
9.6 |
8.4 |
14.9 |
| Headache |
3.2 |
3.1 |
6.9 |
| Weight Increased |
0.5 |
5.2 |
6.4 |
| Back Pain |
4.3 |
2.1 |
5.3 |
| Dizziness |
3.7 |
2.6 |
5.3 |
| Flatulence |
1.6 |
3.7 |
5.3 |
|
|
24
-
Week
Non
-
Controlled
Double
-
Blind
Trial
Adverse
Events
Reported
in
>
5
%
of
Patients
and
More
Commonly
in
Patients
Treated with Pioglitazone Hydrochloride 45 mg + Insulin than in Patients Treated with Pioglitazone Hydrochloride 30 mg + Insulin |
||
|
|
%
of
Patients
|
||
|
|
Pioglitazone
Hydrochloride
30
mg
+
Insulin
N=345 |
Pioglitazone
Hydrochloride
45
mg
+
Insulin
N=345 |
|
| Hypoglycemia |
43.5 |
47.8 |
|
| Edema |
22.0 |
26.1 |
|
| Weight Increased |
7.2 |
13.9 |
|
| Urinary Tract Infection |
4.9 |
8.7 |
|
| Diarrhea |
5.5 |
5.8 |
|
| Back Pain |
3.8 |
6.4 |
|
| Blood Creatine Phosphokinase Increased |
4.6 |
5.5 |
|
| Sinusitis |
4.6 |
5.5 |
|
| Hypertension |
4.1 |
5.5 |
|
A summary of the overall incidence and types of common adverse events reported in the PROactive trial is provided in Table 5. Terms that are reported represent those that occurred at an incidence of >5% and more commonly in patients treated with pioglitazone hydrochloride than in patients who received placebo.
|
Mean duration of patient follow-up was 34.5 months. |
||
|
Table
5
.
PROactive
Trial
:
Incidence
and
Types
of
Adverse
Events
Reported
in
>
5
%
of
Patients
Treated
with
Pioglitazone
Hydrochloride
and
More
Commonly
than
Placebo
|
||
|
|
%
of
Patients
|
|
|
|
Placebo
N=2633 |
Pioglitazone
Hydrochloride
N=2605 |
| Hypoglycemia |
18.8 |
27.3 |
| Edema |
15.3 |
26.7 |
| Cardiac Failure |
6.1 |
8.1 |
| Pain in Extremity |
5.7 |
6.4 |
| Back Pain |
5.1 |
5.5 |
| Chest Pain |
5.0 |
5.1 |
Congestive Heart Failure
A summary of the incidence of adverse events related to congestive heart failure is provided in Table 6 for the 16- to 24-week add-on to sulfonylurea trials, for the 16- to 24-week add-on to insulin trials, and for the 16- to 24-week add-on to metformin trials. None of the events were fatal.
|
Table
6
.
Treatment
-
Emergent
Adverse
Events
of
Congestive
Heart
Failure
(
CHF
)
|
|||||
|
Patients
Treated
with
Pioglitazone
Hydrochloride
or
Placebo
Added
on
to
a
Sulfonylurea
|
|||||
|
|
Number
(%)
of
Patients
|
||||
|
|
Placebo
-
Controlled
Trial
( 16 weeks ) |
Non
-
Controlled
Double
-
Blind
Trial
( 24 weeks ) |
|||
|
|
Placebo
+
Sulfonylurea N=187 |
Pioglitazone
Hydrochloride
15 mg + Sulfonylurea N=184 |
Pioglitazone
Hydrochloride
30 mg + Sulfonylurea N=189 |
Pioglitazone
Hydrochloride
30 mg + Sulfonylurea N=351 |
Pioglitazone
Hydrochloride
45 mg + Sulfonylurea N=351 |
| At least one congestive heart failure event |
2 (1.1%) |
0 |
0 |
1 (0.3%) |
6 (1.7%) |
| Hospitalized |
2 (1.1%) |
0 |
0 |
0 |
2 (0.6%) |
|
Patients
Treated
with
Pioglitazone
Hydrochloride
or
Placebo
Added
on
to
Insulin
|
|||||
|
|
Number
(%)
of
Patients
|
||||
|
|
Placebo
-
Controlled
Trial
( 16 weeks ) |
Non
-
Controlled
Double
-
Blind
Trial
( 24 weeks ) |
|||
|
|
Placebo
+
Insulin
N=187 |
Pioglitazone
Hydrochloride
15 mg + Insulin N=191 |
Pioglitazone
Hydrochloride
30 mg + Insulin N=188 |
Pioglitazone
Hydrochloride
30 mg + Insulin N=345 |
Pioglitazone
Hydrochloride
45 mg + Insulin N=345 |
| At least one congestive heart failure event |
0 |
2 (1.0%) |
2 (1.1%) |
3 (0.9%) |
5 (1.4%) |
| Hospitalized |
0 |
2 (1.0%) |
1 (0.5%) |
1 (0.3%) |
3 (0.9%) |
|
Patients
Treated
with
Pioglitazone
Hydrochloride
or
Placebo
Added on to Metformin |
|||||
|
|
Number
(%)
of
Patients
|
||||
|
|
Placebo
-
Controlled
Trial
( 16 weeks ) |
Non
-
Controlled
Double
-
Blind
Trial
( 24 weeks ) |
|||
|
|
Placebo
+
Metformin N=160 |
Pioglitazone
Hydrochloride
30 mg + Metformin N=168 |
Pioglitazone
Hydrochloride
30 mg + Metformin N=411 |
Pioglitazone
Hydrochloride 45 mg + Metformin N=416 |
|
| At least one congestive heart failure event |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
|
| Hospitalized |
0 |
1 (0.6%) |
0 |
1 (0.2%) |
|
Patients with type 2 diabetes and NYHA class II or early class III congestive heart failure were randomized to receive 24 weeks of double-blind treatment with either pioglitazone hydrochloride at daily doses of 30 mg to 45 mg (n=262) or glyburide at daily doses of 10 mg to 15 mg (n=256). A summary of the incidence of adverse events related to congestive heart failure reported in this study is provided in Table 7.
|
Table
7
.
Treatment
–
Emergent
Adverse
Events
of
Congestive
Heart
Failure
(
CHF
)
in
Patients
with
NYHA
Class
II
or
III
Congestive
Heart
Failure
Treated with Pioglitazone Hydrochloride or Glyburide |
||
|
|
Number
(%)
of
Subjects
|
|
|
|
Pioglitazone
Hydrochloride
N=262 |
Glyburide
N=256 |
| Death due to cardiovascular causes (adjudicated) |
5 (1.9%) |
6 (2.3%) |
| Overnight hospitalization for worsening CHF (adjudicated) |
26 (9.9%) |
12 (4.7%) |
| Emergency room visit for CHF (adjudicated) |
4 (1.5%) |
3 (1.2%) |
| Patients experiencing CHF progression during study |
35 (13.4%) |
21 (8.2%) |
Congestive heart failure events leading to hospitalization that occurred during the PROactive trial are summarized in Table 8.
|
Table
8
.
Treatment
–
Emergent
Adverse
Events
of
Congestive
Heart
Failure
(
CHF
)
in
PROactive
Trial
|
||
|
|
Number
(%)
of
Patients
|
|
|
|
Placebo
N=2633 |
Pioglitazone
Hydrochloride
N=2605 |
| At least one hospitalized congestive heart failure event |
108 (4.1%) |
149 (5.7%) |
| Fatal |
22 (0.8%) |
25 (1.0%) |
| Hospitalized, nonfatal |
86 (3.3%) |
124 (4.7%) |
Cardiovascular Safety
In the PROactive trial, 5238 patients with type 2 diabetes and a history of macrovascular disease were randomized to pioglitazone hydrochloride (N=2605), force-titrated up to 45 mg daily or placebo (N=2633) in addition to standard of care. Almost all patients (95%) were receiving cardiovascular medications (beta blockers, ACE inhibitors, angiotensin II receptor blockers, calcium channel blockers, nitrates, diuretics, aspirin, statins and fibrates). At baseline, patients had a mean age of 62 years, mean duration of diabetes of 9.5 years, and mean HbA1c of 8.1%. Mean duration of follow-up was 34.5 months.
The primary objective of this trial was to examine the effect of pioglitazone hydrochloride on mortality and macrovascular morbidity in patients with type 2 diabetes mellitus who were at high risk for macrovascular events. The primary efficacy variable was the time to the first occurrence of any event in a cardiovascular composite endpoint that included all-cause mortality, nonfatal myocardial infarction (MI) including silent MI, stroke, acute coronary syndrome, cardiac intervention including coronary artery bypass grafting or percutaneous intervention, major leg amputation above the ankle, and bypass surgery or revascularization in the leg. A total of 514 (19.7%) patients treated with pioglitazone hydrochloride and 572 (21.7%) placebo-treated patients experienced at least one event from the primary composite endpoint (hazard ratio 0.90; 95% Confidence Interval: 0.80, 1.02; p=0.10).
Although there was no statistically significant difference between pioglitazone hydrochloride and placebo for the three- year incidence of a first event within this composite, there was no increase in mortality or in total macrovascular events with pioglitazone hydrochloride. The number of first occurrences and total individual events contributing to the primary composite endpoint is shown in Table 9.
|
CABG = coronary artery bypass grafting; PCI = percutaneous intervention |
||||
|
Table
9
.
PROactive
:
Number
of
First
and
Total
Events
for
Each
Component
within
the
Cardiovascular
Composite
Endpoint
|
||||
|
Cardiovascular
Events
|
Placebo
N = 2633 |
Pioglitazone
Hydrochloride
N = 2605 |
||
|
|
First Events n (%) |
Total events n |
First Events n (%) |
Total events n |
| Any event |
572 (21.7) |
900 |
514 (19.7) |
803 |
| All-cause mortality |
122 (4.6) |
186 |
110 (4.2) |
177 |
| Nonfatal myocardial infarction (MI) |
118 (4.5) |
157 |
105 (4.0) |
131 |
| Stroke |
96 (3.6) |
119 |
76 (2.9) |
92 |
| Acute coronary syndrome |
63 (2.4) |
78 |
42 (1.6) |
65 |
| Cardiac intervention (CABG/PCI) |
101 (3.8) |
240 |
101 (3.9) |
195 |
| Major leg amputation |
15 (0.6) |
28 |
9 (0.3) |
28 |
| Leg revascularization |
57 (2.2) |
92 |
71 (2.7) |
115 |
Weight Gain
Dose-related weight gain occurs when pioglitazone hydrochloride is used alone or in combination with other antidiabetic medications. The mechanism of weight gain is unclear but probably involves a combination of fluid retention and fat accumulation.
Tables 10 and 11 summarize the changes in body weight with pioglitazone hydrochloride and placebo in the 16- to 26-week randomized, double-blind monotherapy and 16- to 24-week combination add-on therapy trials and in the PROactive trial.
|
Table
10
.
Weight
Changes
(
kg
)
from
Baseline
During
Randomized , Double - Blind Clinical Trials |
|||||
|
|
Control
Group
( Placebo ) |
Pioglitazone
Hydrochloride
15 mg |
Pioglitazone
Hydrochloride
30 mg |
Pioglitazone
Hydrochloride
45 mg |
|
|
|
Median (25t h/75t h percentile) |
Median (25t h/75t h percentile) |
Median (25t h/75t h percentile) |
Median (25t h/75t h percentile) |
|
|
Monotherapy
( 16 to 26 weeks ) |
|
-1.4 (-2.7/0.0) N=256 |
0.9 (-0.5/3.4) N = 79 |
1.0 (-0.9/3.4) N=188 |
2.6 (0.2/5.4) N = 79 |
|
Combination Therapy |
Sulfonylurea |
-0.5 (-1.8/0.7) N=187 |
2.0 (0.2/3.2) N=183 |
3.1 (1.1/5.4) N=528 |
4.1 (1.8/7.3) N=333 |
|
( 16 to 24 weeks ) |
Metformin |
-1.4 (-3.2/0.3) N=160 |
N/A |
0.9 (-1.3/3.2) N=567 |
1.8 (-0.9/5.0) N=407 |
|
|
Insulin |
0.2 (-1.4/1.4) N=182 |
2.3 (0.5/4.3) N=190 |
3.3 (0.9/6.3) N=522 |
4.1 (1.4/6.8) N=338 |
|
Note: Median exposure for both pioglitazone hydrochloride and Placebo was 2.7 years. |
||
|
Table
11
.
Median
Change
in
Body
Weight
in
Patients
Treated
with
Pioglitazone
Hydrochloride
Versus
Patients
Treated
with
Placebo
During
the
Double
-
Blind
Treatment
Period
in
the
PROactive
Trial
|
||
|
|
Placebo
|
Pioglitazone
Hydrochloride
|
|
|
Median (25t h/75t h percentile) |
Median (25t h/75t h percentile) |
| Change from baseline to final visit (kg) |
-0.5 (-3.3, 2.0) N=2581 |
+3.6 (0.0, 7.5) N=2560 |
Edema
Edema induced from taking pioglitazone hydrochloride is reversible when pioglitazone hydrochloride is discontinued. The edema usually does not require hospitalization unless there is coexisting congestive heart failure. A summary of the frequency and types of edema adverse events occurring in clinical investigations of pioglitazone hydrochloride is provided in Table 12.
|
Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.” |
|||||
|
Table
12
.
Adverse
Events
of
Edema
in
Patients
Treated
with
Pioglitazone
Hydrochloride
|
|||||
|
|
Number
(%)
of
Patients
|
||||
|
|
Placebo
|
Pioglitazone
Hydrochloride
15 mg |
Pioglitazone
Hydrochloride
30 mg |
Pioglitazone
Hydrochloride
45 mg |
|
| Monotherapy (16 to 26 weeks) |
3 (1.2%) N=259 |
2(2.5%) N= 81 |
13 (4.7%) N= 275 |
11 (6.5%) N=169 |
|
| Combined Therapy |
Sulfonylurea |
4 (2.1%) N=187 |
3(1.6%) N=184 |
61 (11.3%) N=540 |
81 (23.1%) N=351 |
| (16 to 24 weeks) |
Metformin |
4 (2.5%) N=160 |
N/A |
34 (5.9%) N=579 |
58 (13.9%) N=416 |
|
|
Insulin |
13(7.0%) N=187 |
24(12.6%) N=191 |
109(20.5%) N=533 |
90 (26.1%) N=345 |
|
Note: The preferred terms of edema peripheral, generalized edema, pitting edema and fluid retention were combined to form the aggregate term of “edema.” |
|
|
Table
13
.
Adverse
Events
of
Edema
in
Patients
in
the
PROactive
Trial
|
|
|
Number
(%)
of
Patients
|
|
|
Placebo
N=2633 |
Pioglitazone
Hydrochloride
N=2605 |
| 419 (15.9%) |
712 (27.3%) |
Hepatic Effects
There has been no evidence of induced hepatotoxicity with pioglitazone hydrochloride in the pioglitazone hydrochloride controlled clinical trial database to date. One randomized, double-blind 3-year trial comparing pioglitazone hydrochloride to glyburide as add-on to metformin and insulin therapy was specifically designed to evaluate the incidence of serum ALT elevation to greater than three times the upper limit of the reference range, measured every eight weeks for the first 48 weeks of the trial then every 12 weeks thereafter. A total of 3/1051 (0.3%) patients treated with pioglitazone hydrochloride and 9/1046 (0.9%) patients treated with glyburide developed ALT values greater than three times the upper limit of the reference range. None of the patients treated with pioglitazone hydrochloride in the pioglitazone hydrochloride controlled clinical trial database to date have had a serum ALT greater than three times times the upper limit of the reference range and a corresponding total bilirubin greater than two times the upper limit of the reference range, a combination predictive of the potential for severe drug-induced liver injury.
Hypoglycemia
In the pioglitazone hydrochloride clinical trials, adverse events of hypoglycemia were reported based on clinical judgment of the investigators and did not require confirmation with fingerstick glucose testing.
In the 16-week add-on to sulfonylurea trial, the incidence of reported hypoglycemia was 3.7% with pioglitazone hydrochloride 30 mg and 0.5% with placebo. In the 16-week add-on to insulin trial, the incidence of reported hypoglycemia was 7.9% with pioglitazone hydrochloride 15 mg, 15.4% with pioglitazone hydrochloride 30 mg, and 4.8% with placebo.
The incidence of reported hypoglycemia was higher with pioglitazone hydrochloride 45 mg compared to pioglitazone hydrochloride 30 mg in both the 24-week add-on to sulfonylurea trial (15.7% vs. 13.4%) and in the 24-week add-on to insulin trial (47.8% vs. 43.5%).
Three patients in these four trials were hospitalized due to hypoglycemia. All three patients were receiving pioglitazone hydrochloride 30 mg (0.9%) in the 24-week add-on to insulin trial. An additional 14 patients reported severe hypoglycemia (defined as causing considerable interference with patient's usual activities) that did not require hospitalization. These patients were receiving pioglitazone hydrochloride 45 mg in combination with sulfonylurea (n=2) or pioglitazone hydrochloride 30 mg or 45 mg in combination with insulin (n=12).
Urinary Bladder Tumors
Tumors were observed in the urinary bladder of male rats in the two-year carcinogenicity study [see Nonclinical Toxicology (13.1)]. In two 3-year trials in which pioglitazone hydrochloride was compared to placebo or glyburide, there were 16/3656 (0.44%) reports of bladder cancer in patients taking pioglitazone hydrochloride compared to 5/3679 (0.14%) in patients not taking pioglitazone hydrochloride. After excluding patients in whom exposure to study drug was less than one year at the time of diagnosis of bladder cancer, there were six (0.16%) cases on pioglitazone hydrochloride and two (0.05%) cases on placebo. There are too few events of bladder cancer to establish causality.
Laboratory Abnormalities
Hematologic Effects
Pioglitazone hydrochloride may cause decreases in hemoglobin and hematocrit. In placebo-controlled monotherapy trials, mean hemoglobin values declined by 2% to 4% in patients treated with pioglitazone hydrochloride compared with a mean change in hemoglobin of -1% to +1% in placebo-treated patients. These changes primarily occurred within the first 4 to 12 weeks of therapy and remained relatively constant thereafter. These changes may be related to increased plasma volume associated with pioglitazone hydrochloride therapy and are not likely to be associated with any clinically significant hematologic effects.
Creatine Phosphokinase
During protocol-specified measurement of serum creatine phosphokinase (CPK) in pioglitazone hydrochloride clinical trials, an isolated elevation in CPK to greater than 10 times the upper limit of the reference range was noted in nine (0.2%) patients treated with pioglitazone hydrochloride (values of 2150 to 11400 IU/L) and in no comparator-treated patients. Six of these nine patients continued to receive pioglitazone hydrochloride, two patients were noted to have the CPK elevation on the last day of dosing and one patient discontinued pioglitazone hydrochloride due to the elevation. These elevations resolved without any apparent clinical sequelae. The relationship of these events to pioglitazone hydrochloride therapy is unknown.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pioglitazone hydrochloride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- New onset or worsening diabetic macular edema with decreased visual acuity [see Warnings and Precautions (5.7)] .
- Fatal and nonfatal hepatic failure [see Warnings and Precautions (5.3)] .
7. DRUG INTERACTIONS
7.1 Strong CYP2C8 Inhibitors
An inhibitor of CYP2C8 (e.g., gemfibrozil) significantly increases the exposure (area under the serum concentration-time curve or AUC) and half-life (t½) of pioglitazone. Therefore, the maximum recommended dose of pioglitazone hydrochloride is 15 mg daily if used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Dosage and Administration (2.3) and Clinical Pharmacology (12.3)].
7.2 CYP2C8 Inducers
An inducer of CYP2C8 (e.g., rifampin) may significantly decrease the exposure (AUC) of pioglitazone. Therefore, if an inducer of CYP2C8 is started or stopped during treatment with pioglitazone hydrochloride, changes in diabetes treatment may be needed based on clinical response without exceeding the maximum recommended daily dose of 45 mg for pioglitazone hydrochloride [see Clinical Pharmacology (12.3)].
8. USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category C.
There are no adequate and well-controlled studies of pioglitazone hydrochloride in pregnant women. Animal studies show increased rates of post-implantation loss, delayed development, reduced fetal weights, and delayed parturition at doses 10 to 40 times the maximum recommended human dose. Pioglitazone hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Clinical Considerations
Abnormal blood glucose concentrations during pregnancy are associated with a higher incidence of congenital anomalies, as well as increased neonatal morbidity and mortality. Most experts recommend the use of insulin during pregnancy to maintain blood glucose concentrations as close to normal as possible for patients with diabetes.
Animal Data
In animal reproductive studies, pregnant rats and rabbits received pioglitazone at doses up to approximately 17 (rat) and 40 (rabbit) times the maximum recommended human oral dose (MRHD) based on body surface area (mg/m2); no teratogenicity was observed. Increases in embryotoxicity (increased postimplantation losses, delayed development, reduced fetal weights, and delayed parturition) occurred in rats that received oral doses approximately 10 or more times the MRHD (mg/m2 basis). No functional or behavioral toxicity was observed in rat offspring. When pregnant rats received pioglitazone during late gestation and lactation, delayed postnatal development, attributed to decreased body weight, occurred in rat offspring at oral maternal doses approximately two or more times the MRHD (mg/m2 basis). In rabbits, embryotoxicity occurred at oral doses approximately 40 times the MRHD (mg/m2 basis).
8.3 Nursing Mothers
It is not known whether pioglitazone hydrochloride is secreted in human milk. Pioglitazone is secreted in the milk of lactating rats. Because many drugs are excreted in human milk, and because of the potential for pioglitazone hydrochloride to cause serious adverse reactions in nursing infants, a decision should be made to discontinue nursing or discontinue pioglitazone hydrochloride, taking into account the importance of pioglitazone hydrochloride to the mother.
8.4 Pediatric Use
Safety and effectiveness of pioglitazone hydrochloride in pediatric patients have not been established.
Pioglitazone hydrochloride is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and congestive heart failure, fractures, and urinary bladder tumors [see Warnings and Precautions (5.1, 5.4, 5.5 and 5.6)].
8.5 Geriatric Use
A total of 92 patients (15.2%) treated with pioglitazone hydrochloride in the three pooled 16- to 26-week double-blind, placebo-controlled, monotherapy, trials were ≥65 years old and two patients (0.3%) were ≥75 years old. In the two pooled 16- to 24-week add-on to sulfonylurea trials, 201 patients (18.7%) treated with pioglitazone hydrochloride were ≥ 65 years old and 19 (1.8%) were ≥ 75 years old. In the two pooled 16- to 24-week add-on to metformin trials, 155 patients (15.5%) treated with pioglitazone hydrochloride were ≥65 years old and 19 (1.9%) were ≥75 years old. In the two pooled 16- to 24-week add-on to insulin trials, 272 patients (25.4%) treated with pioglitazone hydrochloride were ≥65 years old and 22 (2.1%) were ≥75 years old.
In PROactive, 1068 patients (41.0%) treated with pioglitazone hydrochloride were ≥65 years old and 42 (1.6%) were ≥75 years old.
In pharmacokinetic studies with pioglitazone, no significant differences were observed in pharmacokinetic parameters between elderly and younger patients [see Clinical Pharmacology (12.3)].
Although clinical experiences have not identified differences in effectiveness and safety between the elderly (≥65 years) and younger patients, these conclusions are limited by small sample sizes for patients ≥75 years old.
10. OVERDOSAGE
During controlled clinical trials, one case of overdose with pioglitazone hydrochloride was reported. A male patient took 120 mg per day for four days, then 180 mg per day for seven days. The patient denied any clinical symptoms during this period.
In the event of overdosage, appropriate supportive treatment should be initiated according to the patient's clinical signs and symptoms.
11. DESCRIPTION
Pioglitazone tablets, USP are a thiazolidinedione and an agonist for peroxisome proliferator-activated receptor (PPAR) gamma that contains an oral antidiabetic medication: pioglitazone.
Pioglitazone [(±)-5-[[4-[2-(5-ethyl-2-pyridinyl) ethoxy] phenyl] methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown:
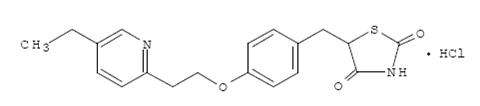
Pioglitazone hydrochloride, USP is an odorless white crystalline powder that has a molecular formula of C19H20N2O3S•HCl and a molecular weight of 392.90 daltons. It is soluble in N,N dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether.
Pioglitazone hydrochloride, USP is available as a tablet for oral administration containing 15 mg, 30 mg, or 45 mg of pioglitazone (as the base) formulated with the following excipients: carboxymethylcellulose calcium, hydroxypropylcellulose, lactose monohydrate, and magnesium stearate.
12. CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Pioglitazone hydrochloride is a thiazolidinedione that depends on the presence of insulin for its mechanism of action. Pioglitazone hydrochloride decreases insulin resistance in the periphery and in the liver resulting in increased insulin-dependent glucose disposal and decreased hepatic glucose output. Pioglitazone is not an insulin secretagogue. Pioglitazone is an agonist for peroxisome proliferator-activated receptor-gamma (PPARγ). PPAR receptors are found in tissues important for insulin action such as adipose tissue, skeletal muscle, and liver. Activation of PPARγ nuclear receptors modulates the transcription of a number of insulin responsive genes involved in the control of glucose and lipid metabolism.
In animal models of diabetes, pioglitazone reduces the hyperglycemia, hyperinsulinemia, and hypertriglyceridemia characteristic of insulin-resistant states such as type 2 diabetes. The metabolic changes produced by pioglitazone result in increased responsiveness of insulin-dependent tissues and are observed in numerous animal models of insulin resistance.
Because pioglitazone enhances the effects of circulating insulin (by decreasing insulin resistance), it does not lower blood glucose in animal models that lack endogenous insulin.
12.2 Pharmacodynamics
Clinical studies demonstrate that pioglitazone hydrochloride improves insulin sensitivity in insulin-resistant patients. Pioglitazone hydrochloride enhances cellular responsiveness to insulin, increases insulin-dependent glucose disposal and improves hepatic sensitivity to insulin. In patients with type 2 diabetes, the decreased insulin resistance produced by pioglitazone hydrochloride results in lower plasma glucose concentrations, lower plasma insulin concentrations, and lower HbA1c values. In controlled clinical trials, pioglitazone hydrochloride had an additive effect on glycemic control when used in combination with a sulfonylurea, metformin, or insulin [see Clinical Studies (14.2)].
Patients with lipid abnormalities were included in clinical trials with pioglitazone hydrochloride. Overall, patients treated with pioglitazone hydrochloride had mean decreases in serum triglycerides, mean increases in HDL cholesterol, and no consistent mean changes in LDL and total cholesterol. There is no conclusive evidence of macrovascular benefit with pioglitazone hydrochloride or any other antidiabetic medication [see Warnings and Precautions (5.9) and Adverse Reactions (6.1)].
In a 26-week, placebo-controlled, dose-ranging monotherapy study, mean serum triglycerides decreased in the 15 mg, 30 mg, and 45 mg pioglitazone hydrochloride dose groups compared to a mean increase in the placebo group. Mean HDL cholesterol increased to a greater extent in patients treated with pioglitazone hydrochloride than in the placebo-treated patients. There were no consistent differences for LDL and total cholesterol in patients treated with pioglitazone hydrochloride compared to placebo (see Table 14).
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||||
|
†p < 0.05 versus placebo |
||||
|
Table
14
.
Lipids
in
a
26
-
Week
Placebo
-
Controlled
Monotherapy
Dose
-
Ranging
Study
|
||||
|
|
Placebo
|
Pioglitazone
Hydrochloride
15
mg
Once Daily |
Pioglitazone
Hydrochloride
30
mg
Once Daily |
Pioglitazone
Hydrochloride
45
mg
Once Daily |
|
Triglycerides
(
mg
/
dL
)
|
N=79 |
N=79 |
N=84 |
N=77 |
| Baseline (mean) |
263 |
284 |
261 |
260 |
| Percent change from baseline (adjusted mean*) |
4.8% |
-9.0%†
|
-9.6%†
|
-9.3%†
|
|
HDL
Cholesterol
(
mg
/
dL
)
|
N=79 |
N=79 |
N=83 |
N=77 |
| Baseline (mean) |
42 |
40 |
41 |
41 |
| Percent change from baseline (adjusted mean*) |
8.1% |
14.1%†
|
12.2% |
19.1%†
|
|
LDL
Cholesterol
(
mg
/
dL
)
|
N=65 |
N=63 |
N=74 |
N=62 |
| Baseline (mean) |
139 |
132 |
136 |
127 |
| Percent change from baseline (adjusted mean*) |
4.8% |
7.2% |
5.2% |
6.0% |
|
Total
Cholesterol
(
mg
/
dL
)
|
N=79 |
N=79 |
N=84 |
N=77 |
| Baseline (mean) |
225 |
220 |
223 |
214 |
| Percent change from baseline (adjusted mean*) |
4.4% |
4.6% |
3.3% |
6.4% |
In the two other monotherapy studies (16 weeks and 24 weeks) and in combination therapy studies with sulfonylurea (16 weeks and 24 weeks), metformin (16 weeks and 24 weeks) or insulin (16 weeks and 24 weeks), the results were generally consistent with the data above.
12.3 Pharmacokinetics
Following once-daily administration of pioglitazone hydrochloride, steady-state serum concentrations of both pioglitazone and its major active metabolites, M-III (keto derivative of pioglitazone) and M-IV (hydroxyl derivative of pioglitazone), are achieved within seven days. At steady-state, M-III and M -IV reach serum concentrations equal to or greater than that of pioglitazone. At steady-state, in both healthy volunteers and patients with type 2 diabetes, pioglitazone comprises approximately 30% to 50% of the peak total pioglitazone serum concentrations (pioglitazone plus active metabolites) and 20% to 25% of the total AUC.
Cmax, AUC, and trough serum concentrations (Cmin) for pioglitazone and M-III and M-IV, increased proportionally with administered doses of 15 mg and 30 mg per day.
Absorption
Following oral administration of pioglitazone, Tmax of pioglitazone was within two hours. Food delays the Tmax to three to four hours but does not alter the extent of absorption (AUC).
Distribution
The mean apparent volume of distribution (Vd/F) of pioglitazone following single-dose administration is 0.63 ± 0.41 (mean ± SD) L/kg of body weight. Pioglitazone is extensively protein bound (> 99%) in human serum, principally to serum albumin. Pioglitazone also binds to other serum proteins, but with lower affinity. M-III and M-IV are also extensively bound (> 98%) to serum albumin.
Metabolism
Pioglitazone is extensively metabolized by hydroxylation and oxidation; the metabolites also partly convert to glucuronide or sulfate conjugates. Metabolites M-III and M-IV are the major circulating active metabolites in humans.
In vitro data demonstrate that multiple CYP isoforms are involved in the metabolism of pioglitazone, which include CYP2C8 and, to a lesser degree, CYP3A4 with additional contributions from a variety of other isoforms including the mainly extrahepatic CYP1A1. In vivo study of pioglitazone in combination with gemfibrozil, a strong CYP2C8 inhibitor, showed that pioglitazone is a CYP2C8 substrate [see Dosage and Administration (2.3) and Drug Interactions (7)]. Urinary 6ß-hydroxycortisol/cortisol ratios measured in patients treated with pioglitazone hydrochloride showed that pioglitazone is not a strong CYP3A4 enzyme inducer.
Excretion and Elimination
Following oral administration, approximately 15% to 30% of the pioglitazone dose is recovered in the urine. Renal elimination of pioglitazone is negligible, and the drug is excreted primarily as metabolites and their conjugates. It is presumed that most of the oral dose is excreted into the bile either unchanged or as metabolites and eliminated in the feces.
The mean serum half-life (t1/2) of pioglitazone and its metabolites (M-III and M-IV) range from three to seven hours and 16 to 24 hours, respectively. Pioglitazone has an apparent clearance, CL/F, calculated to be five to seven L/hr.
Renal Impairment
The serum elimination half-life of pioglitazone, M-III, and M-IV remains unchanged in patients with moderate (creatinine clearance [CLcr] 30 to 50 mL/min) and severe (CLcr < 30 mL/min) renal impairment when compared to subjects with normal renal function. Therefore, no dose adjustment in patients with renal impairment is required.
Hepatic Impairment
Compared with healthy controls, subjects with impaired hepatic function (Child-Turcotte-Pugh Grade B/C) have an approximate 45% reduction in pioglitazone and total pioglitazone (pioglitazone, M-III and M-IV) mean Cmax but no change in the mean AUC values. Therefore, no dose adjustment in patients with hepatic impairment is required.
There are postmarketing reports of liver failure with pioglitazone hydrochloride and clinical trials have generally excluded patients with serum ALT >2.5 times the upper limit of the reference range. Use caution in patients with liver disease [see Warnings and Precautions (5.3)].
Geriatric Patients
In healthy elderly subjects, Cmax of pioglitazone was not significantly different, but AUC values were approximately 21% higher than those achieved in younger subjects. The mean t1/2 of pioglitazone was also prolonged in elderly subjects (about ten hours) as compared to younger subjects (about seven hours). These changes were not of a magnitude that would be considered clinically relevant.
Pediatric Patients
Safety and efficacy of pioglitazone in pediatric patients have not been established. Pioglitazone hydrochloride is not recommended for use in pediatric patients [see Use in Specific Populations (8.4)].
Gender
The mean Cmax and AUC values of pioglitazone were increased 20% to 60% in women compared to men. In controlled clinical trials, HbA1c decreases from baseline were generally greater for females than for males (average mean difference in HbA1c 0.5%). Because therapy should be individualized for each patient to achieve glycemic control, no dose adjustment is recommended based on gender alone.
Ethnicity
Pharmacokinetic data among various ethnic groups are not available.
Drug-Drug Interactions
|
*Daily for 7 days unless otherwise noted |
|||||
|
†% change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively |
|||||
|
‡Pioglitazone had no clinically significant effect on prothrombin time |
|||||
|
Table
15
.
Effect
of
Pioglitazone
Coadministration
on
Systemic
Exposure
of
Other
Drugs
|
|||||
|
Coadministered
Drug
|
|||||
|
Pioglitazone
Dosage
Regimen
(
mg
)*
|
Name
and
Dose
Regimens
|
Change
in
AUC
†
|
Change
in
Cm
a
x
†
|
||
| 45 mg |
Warfarin
‡
|
||||
| (N = 12) |
Daily loading then maintenance doses based PT and INR values |
R-Warfarin |
↓3% |
R-Warfarin |
↓2% |
|
|
Quick's Value = 35 ± 5% |
S-Warfarin |
↓1% |
S-Warfarin |
↑ 1% |
| 45 mg |
Digoxin
|
||||
| (N = 12) |
0.200 mg twice daily (loading dose) then 0.250 mg daily (maintenance dose, 7 days) |
↑15% |
↑17% |
||
| 45 mg daily |
Oral
Contraceptive
|
||||
|
|
[Ethinyl Estradiol (EE) 0.035 mg |
EE |
↓11% |
EE |
↓13% |
| for 21 days (N = 35) |
plus Norethindrone (NE) 1 mg] for 21 days |
NE |
↑3% |
NE |
↓7% |
| 45 mg |
Fexofenadine
|
||||
| (N = 23) |
60 mg twice daily for 7 days |
↑30% |
↑37% |
||
| 45 mg (N = 14) |
Glipizide
|
||||
|
|
5 mg daily for 7 days |
↓3% |
↓8% |
||
| 45 mg daily |
Metformin
|
||||
| for 8 days (N = 16) |
1000 mg single dose on Day 8 |
↓3% |
↓5% |
||
| 45 mg |
Midazolam
|
||||
| (N = 21) |
7.5 mg single dose on Day 15 |
↓26% |
↓26% |
||
| 45 mg |
Ranitidine
|
||||
| (N = 24) |
150 mg twice daily for 7 days |
↑1% |
↓1% |
||
| 45 mg daily for 4 days |
Nifedipine
ER
|
||||
| (N = 24) |
30 mg daily for 4 days |
↓13% |
↓17% |
||
| 45 mg |
Atorvastatin
Ca
|
||||
| (N = 25) |
80 mg daily for 7 days |
↓14% |
↓23% |
||
| 45 mg (N = 22) |
Theophylline
|
||||
|
|
400 mg twice daily for 7 days |
↑2% |
↑5% |
||
|
*Daily for 7 days unless otherwise noted |
|||
|
†Mean ratio (with/without coadministered drug and no change = 1-fold) % change (with/without coadministered drug and no change = 0%); symbols of ↑ and ↓ indicate the exposure increase and decrease, respectively |
|||
|
‡The half-life of pioglitazone increased from 8.3 hours to 22.7 hours in the presence of gemfibrozil [see
Dosage
and
Administration
(
2
.
3
)
and
Drug
Interactions
(
7)] |
|||
|
|
|||
|
Table
16
.
Effect
of
Coadministered
Drugs
on
Pioglitazone
Systemic Exposure |
|||
|
|
Pioglitazone
|
||
|
Coadministered
Drug
and
Dosage
Regimen
|
Dose
Regimen
(
mg
)*
|
Change
in AUC † |
Change
in Cm a x † |
| Gemfibrozil 600 mg twice daily for 2 days (N = 12) |
15 mg single dose |
↑ 3.2-fold‡
|
↑ 6% |
| Ketoconazole 200 mg twice daily for 7 days (N = 28) |
45 mg |
↑34% |
↑14% |
| Rifampin 600 mg daily for 5 days (N = 10) |
30 mg single dose |
↓ 54% |
↓ 5% |
| Fexofenadine 60 mg twice daily for 7 days (N = 23) |
45 mg |
↑ 1% |
0% |
| Ranitidine 150 mg twice daily for 4 days (N = 23) |
45 mg |
↓ 13% |
↓ 16% |
| Nifedipine ER 30 mg daily for 7 days (N = 23) |
45 mg |
↑ 5% |
↑ 4% |
| Atorvastatin Ca 80 mg daily for 7 days (N = 24) |
45 mg |
↓ 24% |
↓ 31% |
| Theophylline 400 mg twice daily for 7 days (N = 22) |
45 mg |
↓ 4% |
↓ 2% |
13. NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A two-year carcinogenicity study was conducted in male and female rats at oral doses up to 63 mg/kg (approximately 14 times the maximum recommended human oral dose of 45 mg based on mg/ m2). Drug-induced tumors were not observed in any organ except for the urinary bladder of male rats. Benign and/or malignant transitional cell neoplasms were observed in male rats at 4 mg/kg/day and above (approximately equal to the maximum recommended human oral dose based on mg/m2). Urinary calculi with subsequent irritation and hyperplasia were postulated as the mechanism for bladder tumors observed in male rats. A two-year mechanistic study in male rats utilizing dietary acidification to reduce calculi formation was completed in 2009. Dietary acidification decreased but did not abolish the hyperplastic changes in the bladder. The presence of calculi exacerbated the hyperplastic response to pioglitazone but was not considered the primary cause of the hyperplastic changes.
The relevance to humans of the bladder findings in the male rat cannot be excluded.
A two-year carcinogenicity study was also conducted in male and female mice at oral doses up to 100 mg/kg/day (approximately 11 times the maximum recommended human oral dose based on mg/ m2). No drug-induced tumors were observed in any organ.
Pioglitazone hydrochloride was not mutagenic in a battery of genetic toxicology studies, including the Ames bacterial assay, a mammalian cell forward gene mutation assay (CHO/HPRT and AS52/XPRT), an in vitro cytogenetics assay using CHL cells, an unscheduled DNA synthesis assay, and an in vivo micronucleus assay.
No adverse effects upon fertility were observed in male and female rats at oral doses up to 40 mg/kg pioglitazone hydrochloride daily prior to and throughout mating and gestation (approximately nine times the maximum recommended human oral dose based on mg/m2).
13.2 Animal Toxicology and/or Pharmacology
Heart enlargement has been observed in mice (100 mg/kg), rats (4 mg/kg and above) and dogs (3 mg/kg) treated orally with pioglitazone hydrochloride (approximately 11, 1, and 2 times the maximum recommended human oral dose for mice, rats, and dogs, respectively, based on mg/m2). In a one-year rat study, drug-related early death due to apparent heart dysfunction occurred at an oral dose of 160 mg/kg/day (approximately 35 times the maximum recommended human oral dose based on mg/m2). Heart enlargement was seen in a 13-week study in monkeys at oral doses of 8.9 mg/kg and above (approximately four times the maximum recommended human oral dose based on mg/m2), but not in a 52-week study at oral doses up to 32 mg/kg (approximately 13 times the maximum recommended human oral dose based on mg/m2).
14. CLINICAL STUDIES
14.1 Monotherapy
Three randomized, double-blind, placebo-controlled trials with durations from 16 to 26 weeks were conducted to evaluate the use of pioglitazone hydrochloride as monotherapy in patients with type 2 diabetes. These trials examined pioglitazone hydrochloride at doses up to 45 mg or placebo once daily in a total of 865 patients.
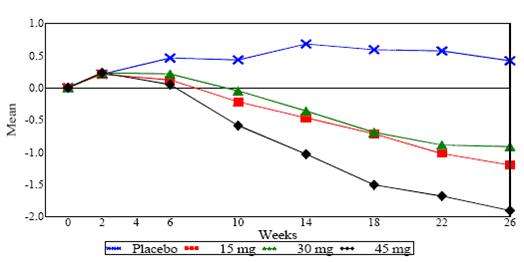
In a 26-week dose-ranging monotherapy trial, 408 patients with type 2 diabetes were randomized to receive 7.5 mg, 15 mg, 30 mg, or 45 mg of pioglitazone hydrochloride, or placebo once daily. Therapy with any previous antidiabetic agent was discontinued eight weeks prior to the double-blind period. Treatment with 15 mg, 30 mg, and 45 mg of pioglitazone hydrochloride produced statistically significant improvements in HbA1c and fasting plasma glucose (FPG) at endpoint compared to placebo (see Figure 1, Table 17).
Figure 1 shows the time course for changes in HbA1c in this 26-week study.
Figure 1. Mean Change from Baseline for HbA1c in a 26-Week Placebo-Controlled Dose-Ranging Study (Observed Values)
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||||
|
†p ≤ 0.05 vs. placebo |
||||
|
Table
17
.
Glycemic
Parameters
in
a
26
-
Week
Placebo
-
Controlled
Dose - Ranging Monotherapy Trial |
||||
|
|
Placebo
|
Pioglitazone
Hydrochloride
15
mg
Once Daily |
Pioglitazone
Hydrochloride
30
mg
Once Daily |
Pioglitazone
Hydrochloride
45
mg
Once Daily |
|
Total
Population
|
|
|
|
|
|
HbA1c
(%)
|
N=79 |
N=79 |
N=85 |
N=76 |
| Baseline (mean) |
10.4 |
10.2 |
10.2 |
10.3 |
| Change from baseline (adjusted mean*) |
0.7 |
-0.3 |
-0.3 |
-0.9 |
| Difference from placebo (adjusted mean*) 95% Confidence Interval |
|
-1.0†
(-1.6, -0.4) |
-1.0† (-1.6, -0.4) |
-1.6† (-2.2, -1.0) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=79 |
N=79 |
N=84 |
N=77 |
| Baseline (mean) |
268 |
267 |
269 |
276 |
| Change from baseline (adjusted mean*) |
9 |
-30 |
-32 |
-56 |
| Difference from placebo (adjusted mean*) 95% Confidence Interval |
|
-39† (-63, -16) |
-41†
(-64, -18) |
-65†
(-89, -42) |
In a 24-week placebo-controlled monotherapy trial, 260 patients with type 2 diabetes were randomized to one of two forced-titration pioglitazone hydrochloride treatment groups or a mock-titration placebo group. Therapy with any previous antidiabetic agent was discontinued six weeks prior to the double-blind period. In one pioglitazone hydrochloride treatment group, patients received an initial dose of 7.5 mg once daily. After four weeks, the dose was increased to 15 mg once daily and after another four weeks, the dose was increased to 30 mg once daily for the remainder of the trial (16 weeks). In the second pioglitazone hydrochloride treatment group, patients received an initial dose of 15 mg once daily and were titrated to 30 mg once daily and 45 mg once daily in a similar manner. Treatment with pioglitazone hydrochloride, as described, produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo (see Table 18).
|
* Final dose in forced titration |
|||
|
†Adjusted for baseline, pooled center, and pooled center by treatment interaction |
|||
|
‡p ≤ 0.05 vs. placebo |
|||
|
Table
18
.
Glycemic
Parameters
in
a
24
-
Week
Placebo
-
Controlled
Forced - Titration Monotherapy Trial |
|||
|
|
Placebo
|
Pioglitazone
Hydrochloride
30 mg * Once Daily |
Pioglitazone
Hydrochloride
45 mg * Once Daily |
|
Total
Population
|
|||
|
HbA1c
(%)
|
N=83 |
N=85 |
N=85 |
| Baseline (mean) |
10.8 |
10.3 |
10.8 |
| Change from baseline (adjusted mean†) |
0.9 |
-0.6 |
-0.6 |
| Difference from placebo (adjusted mean†) 95% Confidence Interval |
|
-1.5‡
(-2.0, -1.0) |
-1.5‡
(-2.0, -1.0) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=78 |
N=82 |
N=85 |
| Baseline (mean) |
279 |
268 |
281 |
| Change from baseline (adjusted mean†) |
18 |
-44 |
-50 |
| Difference from placebo (adjusted mean†) 95% Confidence Interval |
|
-62‡
(-82, -0.41) |
-68‡
(-88, -0.48) |
In a 16-week monotherapy trial, 197 patients with type 2 diabetes were randomized to treatment with 30 mg of pioglitazone hydrochloride or placebo once daily. Therapy with any previous antidiabetic agent was discontinued six weeks prior to the double-blind period. Treatment with 30 mg of pioglitazone hydrochloride produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo (see Table 19).
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||
|
†p ≤ 0.050 vs. placebo |
||
|
Table
19
.
Glycemic
Parameters
in
a
16
-
Week
Placebo
-
Controlled
Monotherapy
Trial
|
||
|
|
Placebo
|
Pioglitazone
Hydrochloride
30
mg
Once Daily |
|
Total
Population
|
||
|
HbA1c
(%)
|
N=93 |
N=100 |
| Baseline (mean) |
10.3 |
10.5 |
| Change from baseline (adjusted mean*) |
0.8 |
-0.6 |
| Difference from placebo (adjusted mean*) 95% Confidence Interval |
|
-1.4† (-1.8, -0.9) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=91 |
N=99 |
| Baseline (mean) |
270 |
273 |
| Change from baseline (adjusted mean*) |
8 |
-50 |
| Difference from placebo (adjusted mean*) 95% Confidence Interval |
|
-58† (-77, -38) |
14.2 Combination Therapy
Three 16-week, randomized, double-blind, placebo-controlled clinical trials were conducted to evaluate the effects of pioglitazone hydrochloride (15 mg and/or 30 mg) on glycemic control in patients with type 2 diabetes who were inadequately controlled (HbA1c ≥8%) despite current therapy with a sulfonylurea, metformin, or insulin. In addition, three 24-week randomized, double-blind clinical trials were conducted to evaluate the effects of pioglitazone hydrochloride 30 mg vs. pioglitazone hydrochloride 45 mg on glycemic control in patients with type 2 diabetes who were inadequately controlled (HbA1c ≥8%) despite current therapy with a sulfonylurea, metformin, or insulin. Previous diabetes treatment may have been monotherapy or combination therapy.
Add-on to Sulfonylurea Trials
Two clinical trials were conducted with pioglitazone hydrochloride in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone hydrochloride or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone hydrochloride as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to sulfonylurea (see Table 20).
|
*Adjusted for baseline, pooled center, and pooled center by treatment interaction |
|||
|
†p ≤ 0.05 vs. placebo + sulfonylurea |
|||
|
Table
20
.
Glycemic
Parameters
in
a
16
-
Week
Placebo
-
Controlled
,
Add
-
on
to
Sulfonylurea
Trial
|
|||
|
|
Placebo
+
Sulfonylurea
|
Pioglitazone
Hydrochloride
15
mg
+
Sulfonylurea
|
Pioglitazone
Hydrochloride
30
mg
+
Sulfonylurea
|
|
Total
Population
|
|||
|
HbA1c
(%)
|
N=181 |
N=176 |
N=182 |
| Baseline (mean) |
9.9 |
10.0 |
9.9 |
| Change from baseline (adjusted mean*) |
0.1 |
-0.8 |
-1.2 |
| Difference from placebo + sulfonylurea (adjusted mean*) 95% Confidence Interval |
|
-0.9† (-1.2, -0.6) |
-1.3†
(-1.6, -1.0) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=182 |
N=179 |
N=186 |
| Baseline (mean) |
236 |
247 |
239 |
| Change from baseline (adjusted mean*) |
6 |
-34 |
-52 |
| Difference from placebo + sulfonylurea (adjusted mean*) 95% Confidence Interval |
|
-39† (-52, -27) |
-58†
(-70, -46) |
In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone hydrochloride once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 21). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose.
The therapeutic effect of pioglitazone hydrochloride in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose.
|
95% CI = 95% confidence interval |
||
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||
|
Table
21
.
Glycemic
Parameters
in
a
24
-
Week
Add
-
on
to
Sulfonylurea
Trial
|
||
|
|
Pioglitazone
Hydrochloride
30
mg
+
Sulfonylurea
|
Pioglitazone
Hydrochloride
45
mg
+
Sulfonylurea
|
|
Total
Population
|
||
|
HbA1c
(%)
|
N=340 |
N=332 |
| Baseline (mean) |
9.8 |
9.9 |
| Change from baseline (adjusted mean*) |
-1.6 |
-1.7 |
| Difference from 30 mg daily pioglitazone hydrochloride + sulfonylurea (adjusted mean*) (95% CI) |
|
- 0.1 (-0.4, 0.1) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=338 |
N=329 |
| Baseline (mean) |
214 |
217 |
| Change from baseline (adjusted mean*) |
-52 |
-56 |
| Difference from 30 mg daily pioglitazone hydrochloride + sulfonylurea (adjusted mean*) (95% CI) |
|
-5 (-12, 3) |
Add-on to Metformin Trials
Two clinical trials were conducted with pioglitazone hydrochloride in combination with metformin. Both trials included patients with type 2 diabetes on any dose of metformin, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first trial, 328 patients were randomized to receive either 30 mg of pioglitazone hydrochloride or placebo once daily for 16 weeks in addition to their current metformin regimen. Treatment with pioglitazone hydrochloride as add-on to metformin produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to metformin (see Table 22).
|
*Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||
|
†p ≤ 0.05 vs. placebo + metformin |
||
|
Table
22
.
Glycemic
Parameters
in
a
16
-
Week
Placebo
-
Controlled
,
Add
-
on
to
Metformin
Trial
|
||
|
|
Placebo
+
Metformin
|
Pioglitazone
Hydrochloride
30
mg
+
Metformin
|
|
Total
Population
|
||
|
HbA1c
(%)
|
N=153 |
N=161 |
| Baseline (mean) |
9.8 |
9.9 |
| Change from baseline (adjusted mean*) |
0.2 |
-0.6 |
| Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval |
|
-0.8† (-1.2, -0.5) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=157 |
N=165 |
| Baseline (mean) |
260 |
254 |
| Change from baseline (adjusted mean*) |
-5 |
-43 |
| Difference from placebo + metformin (adjusted mean*) 95% Confidence Interval |
|
-38† (-49, -26) |
In the second trial, 827 patients were randomized to receive either 30 mg or 45 mg of pioglitazone hydrochloride once daily for 24 weeks in addition to their current metformin regimen. The mean reduction from baseline at Week 24 in HbA1c was 0.8% for the 30 mg dose and 1.0% for the 45 mg dose (see Table 23). The mean reduction from baseline at Week 24 in FPG was 38 mg/dL for the 30 mg dose and 51 mg/dL for the 45 mg dose.
|
95% CI = 95% confidence interval |
||
|
*Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||
|
†p ≤ 0.05 vs. 30 mg daily pioglitazone hydrochloride + metformin |
||
|
Table
23
.
Glycemic
Parameters
in
a
24
-
Week
Add
-
on
to
Metformin
Study
|
||
|
|
Pioglitazone
Hydrochloride
30
mg
+
Metformin
|
Pioglitazone
Hydrochloride
45
mg
+
Metformin
|
|
Total
Population
|
||
|
HbA1C
(%)
|
N=400 |
N=398 |
| Baseline (mean) |
9.9 |
9.8 |
| Change from baseline (adjusted mean*) |
-0.8 |
-1.0 |
| Difference from 30 mg daily pioglitazone hydrochloride + Metformin (adjusted mean*) (95% CI) |
|
-0.2 (-0.5, 0.1) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=398 |
N=399 |
| Baseline (mean) |
233 |
232 |
| Change from baseline (adjusted mean*) |
-38 |
-51 |
| Difference from 30 mg daily pioglitazone hydrochloride + Metformin (adjusted mean*) (95% CI) |
|
-12† (-21, -4) |
The therapeutic effect of pioglitazone hydrochloride in combination with metformin was observed in patients regardless of the metformin dose.
Add-on to Insulin Trials
Two clinical trials were conducted with pioglitazone hydrochloride in combination with insulin. Both trials included patients with type 2 diabetes on insulin, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn prior to starting study treatment. In the first trial, 566 patients were randomized to receive either 15 mg or 30 mg of pioglitazone hydrochloride or placebo once daily for 16 weeks in addition to their insulin regimen. Treatment with pioglitazone hydrochloride as add-on to insulin produced statistically significant improvements in HbA1c and FPG at endpoint compared to placebo add-on to insulin (see Table 24). The mean daily insulin dose at baseline in each treatment group was approximately 70 units. The majority of patients (75% overall, 86% treated with placebo, 77% treated with pioglitazone hydrochloride 15 mg, and 61% treated with pioglitazone hydrochloride 30 mg) had no change in their daily insulin dose from baseline to the final study visit. The mean change from baseline in daily dose of insulin (including patients with no insulin dose modifications) was -3 units in the patients treated with pioglitazone hydrochloride 15 mg, -8 units in the patients treated with pioglitazone hydrochloride 30 mg, and -1 unit in patients treated with placebo.
|
*Adjusted for baseline, pooled center, and pooled center by treatment interaction |
|||
|
†p ≤ 0.05 vs. placebo + insulin |
|||
|
Table
24
.
Glycemic
Parameters
in
a
16
-
Week
Placebo
-
Controlled
,
Add
-
on
to
Insulin
Trial
|
|||
|
|
Placebo
+
Insulin
|
Pioglitazone
Hydrochloride
15
mg
+
Insulin
|
Pioglitazone
Hydrochloride
30
mg
+
Insulin
|
|
Total
Population
|
|||
|
HbA1C
(%)
|
N=177 |
N=177 |
N=185 |
| Baseline (mean) |
9.8 |
9.8 |
9.8 |
| Change from baseline (adjusted mean*) |
-0.3 |
-1.0 |
-1.3 |
| Difference from placebo + Insulin (adjusted mean*) 95% Confidence Interval |
|
-0.7† (-1.0, -0.5) |
-1.0† (-1.3, -0.7) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=179 |
N=183 |
N=184 |
| Baseline (mean) |
221 |
222 |
229 |
| Change from baseline (adjusted mean*) |
1 |
-35 |
-48 |
| Difference from placebo + Insulin (adjusted mean*) 95% Confidence Interval |
|
-35† (-51, -19) |
-49† (-65, -33) |
In the second trial, 690 patients receiving a median of 60 units per day of insulin were randomized to receive either 30 mg or 45 mg of pioglitazone hydrochloride once daily for 24 weeks in addition to their current insulin regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.2% for the 30 mg dose and 1.5% for the 45 mg dose. The mean reduction from baseline at Week 24 in FPG was 32 mg/dL for the 30 mg dose and 46 mg/dL for the 45 mg dose (see Table 25). The mean daily insulin dose at baseline in both treatment groups was approximately 70 units. The majority of patients (55% overall, 58% treated with pioglitazone hydrochloride 30 mg, and 52% treated with pioglitazone hydrochloride 45 mg) had no change in their daily insulin dose from baseline to the final study visit. The mean change from baseline in daily dose of insulin (including patients with no insulin dose modifications) was -5 units in the patients treated with pioglitazone hydrochloride 30 mg and -8 units in the patients treated with pioglitazone hydrochloride 45 mg.
The therapeutic effect of pioglitazone hydrochloride in combination with insulin was observed in patients regardless of the insulin dose.
|
95% CI = 95% confidence interval |
||
|
* Adjusted for baseline, pooled center, and pooled center by treatment interaction |
||
|
†p ≤ 0.05 vs. 30 mg daily pioglitazone hydrochloride + insulin |
||
|
Table
25
.
Glycemic
Parameters
in
a
24
-
Week
Add
-
on
to
Insulin
Trial
|
||
|
|
Pioglitazone
Hydrochloride
30
mg
+
Insulin
|
Pioglitazone
Hydrochloride
45
mg
+
Insulin
|
|
Total
Population
|
||
|
HbA1c
(%)
|
N=328 |
N=328 |
| Baseline (mean) |
9.9 |
9.7 |
| Change from baseline (adjusted mean*) |
-1.2 |
-1.5 |
| Difference from 30 mg daily pioglitazone hydrochloride + Insulin (adjusted mean*) (95% CI) |
|
-0.3† (-0.5, -0.1) |
|
Fasting
Plasma
Glucose
(
mg
/
dL
)
|
N=325 |
N=327 |
| Baseline (mean) |
202 |
199 |
| Change from baseline (adjusted mean*) |
-32 |
-46 |
| Difference from 30 mg daily pioglitazone hydrochloride + Insulin (adjusted mean*) (95% CI) |
|
-14† (-25, -3) |
16. HOW SUPPLIED/STORAGE AND HANDLING
Pioglitazone hydrochloride, USP is available in 15 mg, 30 mg, and 45 mg tablets as follows:
15 mg Tablet: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '140' on one side and '15' on other side, available in:
Bottles of 30 NDC 13668-140-30
Bottles of 90 NDC 13668-140-90
Bottles of 500 NDC 13668-140-05
Bottles of 9000 NDC 13668-140-53
100 Unit Dose Tablets NDC 13668-140-74
30 mg Tablet: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '1119' on one side and plain on other side, available in:
Bottles of 30 NDC 13668-119-30
Bottles of 90 NDC 13668-119-90
Bottles of 500 NDC 13668-119-05
Bottles of 7000 NDC 13668-119-52
100 Unit Dose Tablets NDC 13668-119-74
45 mg Tablet: White to off-white, round, flat faced beveled edge, uncoated tablets debossed with '1120' on one side and plain on other side, available in:
Bottles of 30 NDC 13668-120-30
Bottles of 90 NDC 13668-120-90
Bottles of 500 NDC 13668-120-05
Bottles of 5000 NDC 13668-120-51
100 Unit Dose Tablets NDC 13668-120-74
Storage
Store at 20°-25°C (68°-77°F); excursions permitted to 15-30°C (59°-86°F) [see USP Controlled Room Temperature]. Keep container tightly closed, and protect from moisture.
17. PATIENT COUNSELING INFORMATION
See FDA-Approved Patient Labeling (Medication Guide).
- It is important to instruct patients to adhere to dietary instructions and to have blood glucose and glycosylated hemoglobin tested regularly. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be reminded to seek medical advice promptly.
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on pioglitazone tablets should immediately report these symptoms to a physician.
- Tell patients to promptly stop taking pioglitazone tablets and seek immediate medical advice if there is unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine as these symptoms may be due to hepatotoxicity.
- Tell patients to promptly report any sign of macroscopic hematuria or other symptoms such as dysuria or urinary urgency that develop or increase during treatment as these may be due to bladder cancer.
- Tell patients to take pioglitazone tablets once daily. Pioglitazone tablets can be taken with or without meals. If a dose is missed on one day, the dose should not be doubled the following day.
- When using combination therapy with insulin or other antidiabetic medications, the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and their family members.
- Inform patients that therapy with pioglitazone tablets, like other thiazolidinediones, may result in ovulation in some premenopausal anovulatory women. As a result, these patients may be at an increased risk for pregnancy while taking pioglitazone tablets. Therefore, adequate contraception should be recommended for all premenopausal women who are prescribed pioglitazone tablets.
Licensed - United States Patents Nos. 5,965,584, 6,150,383, 6,150,384, 6,166,042, 6,166,043, 6,172,090, 6,211,205, 6,271,243, 6,329,404, and 6,303,640.

Manufactured by:
TORRENT PHARMACEUTICALS LTD., Indrad-382 721, Dist. Mehsana, INDIA.
For:
TORRENT PHARMA INC., 150 Allen Road, Suite 102, Basking Ridge, NJ 07920
8044139 Revised December 2013
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pioglitazone Tablets 15mg

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pioglitazone Tablets 30mg

PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Pioglitazone Tablets 45mg

PIOGLITAZONEHYDROCHLORIDEPIOGLITAZONEHYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||