PHENYTOIN SODIUM
PHENYTOIN SODIUM INJECTION, USP
FULL PRESCRIBING INFORMATION
This drug must be administered slowly. In adults do not exceed 50 mg per minute intravenously. In neonates, the drug should be administered at a rate not exceeding 1 to 3 mg/kg/min.
Phenytoin sodium injection, USP is a sterile solution for intravenous (no infusion) or intramuscular use, containing in each mL phenytoin sodium 50 mg, propylene glycol 0.4 mL and alcohol 0.1 mL in Water for Injection. pH 10.0 to 12.3; sodium hydroxide added, if needed, for pH adjustment.
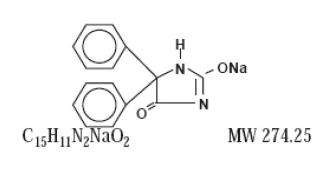
Phenytoin sodium is related to the barbiturates in chemical structure, but has a five-membered ring. The chemical name is sodium 5,5-diphenyl-2,4-imidazolidinedione. The structural formula is as follows:
Phenytoin is an anticonvulsant which may be useful in the treatment of status epilepticus of the grand mal type. The primary site of action appears to be the motor cortex where spread of seizure activity is inhibited. Possibly by promoting sodium efflux from neurons, phenytoin tends to stabilize the threshold against hyperexcitability caused by excessive stimulation or environmental changes capable of reducing membrane sodium gradient. This includes the reduction of posttetanic potentiation at synapses. Loss of posttetanic potentiation prevents cortical seizure foci from detonating adjacent cortical areas. Phenytoin reduces the maximal activity of brain stem centers responsible for the tonic phase of grand mal seizures.
The plasma half-life in man after intravenous administration ranges from 10 to 15 hours. Optimum control without clinical signs of toxicity occurs most often with serum levels between 10 and 20 mcg/mL.
A fall in plasma levels may occur when patients are changed from oral to intramuscular administration. The drop is caused by slower absorption, as compared to oral administration, due to the poor water solubility of phenytoin. Intravenous administration is the preferred route for producing rapid therapeutic serum levels.
There are occasions when intramuscular administration may be required, i.e., postoperatively, in comatose patients, for GI upsets. During these periods, a sufficient dose must be administered intramuscularly to maintain the plasma level within the therapeutic range. Where oral dosage is resumed following intramuscular usage, the oral dose should be properly adjusted to compensate for the slow, continuing IM absorption to avoid toxic symptoms.
Patients stabilized on a daily oral regimen of phenytoin experience a drop in peak blood levels to 50 to 60 percent of stable levels if crossed over to an equal dose administered intramuscularly. However, the intramuscular depot of poorly soluble material is eventually absorbed, as determined by urinary excretion of
5(p-hydroxyphenyl)5-phenylhydantoin (HPPH), the principal metabolite, as well as the total amount of drug eventually appearing in the blood.
A short-term (one week) study indicates that patients do not experience the expected drop in blood levels when crossed over to the intramuscular route if the phenytoin IM dose is increased by 50 percent over the previously established oral dose. To avoid drug cumulation due to absorption from the muscle depots, it is recommended that for the first week back on oral phenytoin, the dose be reduced to half of the original oral dose (one third of the IM dose). Experience for the periods greater than one week is lacking and blood level monitoring is recommended. For administration of phenytoin in patients who cannot take oral medication for periods greater than a week, gastric intubation may be considered.
Phenytoin sodium injection is indicated for the control of status epilepticus of the grand mal type, and prevention and treatment of seizures occurring during neurosurgery.
Phenytoin is contraindicated in patients with a history of hypersensitivity to hydantoin products. Because of its effect on ventricular automaticity, phenytoin is contraindicated in sinus bradycardia, sino atrial block, second and third degree A-V block, and patients with Adams-Stokes syndrome.
Intravenous administration should not exceed 50 mg per minute in adults.
In neonates, the drug should be administered at a rate not exceeding 1 to 3 mg/kg/min.
Severe cardiotoxic reactions and fatalities have been reported with atrial and ventricular conduction depression and ventricular fibrillation. Severe complications are most commonly encountered in elderly or gravely ill patients.
Phenytoin should be used with caution in patients with hypotension and severe myocardial insufficiency.
Hypotension usually occurs when the drug is administered rapidly by the intravenous route.
The intramuscular route is not recommended for the treatment of status epilepticus since blood levels of phenytoin in the therapeutic range cannot be readily achieved with doses and methods of administration ordinarily employed.
There have been a number of reports suggesting a relationship between phenytoin and the development of lymphadenopathy (local or generalized) including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin’s Disease. Although a cause and effect relationship has not been established, the occurrence of lymphadenopathy indicates the need to differentiate such a condition from other types of lymph node pathology. Lymph node involvement may occur with or without symptoms and signs resembling serum sickness, e.g., fever, rash, and liver involvement.
In all cases of lymphadenopathy, follow up observation for an extended period is indicated and every effort should be made to achieve seizure control using alternative antiepileptic drugs.
Acute alcoholic intake may increase phenytoin serum levels while chronic alcoholic use may decrease serum levels.
Usage in Pregnancy
A number of reports suggest an association between the use of antiepileptic drugs by women with epilepsy and a higher incidence of birth defects in children born to these women. Data are more extensive with respect to phenytoin and phenobarbital, but these are also the most commonly prescribed antiepileptic drugs; less systematic or anecdotal reports suggest a possible similar association with the uses of all known antiepileptic drugs.
The reports suggesting a higher incidence of birth defects in children of drug treated epileptic women cannot be regarded as adequate to prove a definite cause and effect relationship. There are intrinsic methodologic problems in obtaining adequate data on drug teratogenicity in humans; genetic factors or the epileptic condition itself may be more important than drug therapy in leading to birth defects. The great majority of mothers on antiepileptic medication deliver normal infants.
It is important to note that antiepileptic drugs should not be discontinued in patients in whom the drug is administered to prevent major seizures, because of the strong possibility of precipitating status epilepticus with attendant hypoxia and threat to life. In individual cases where the severity and frequency of the seizure disorder are such that the removal of medication does not pose a serious threat to the patient, discontinuation of the drug may be considered prior to and during pregnancy, although it cannot be said with any confidence that even minor seizures do not pose some hazard to the developing embryo or fetus. The prescribing physician will wish to weigh these considerations in treating or counseling epileptic women of childbearing potential.
In addition to the reports of increased incidence of congenital malformation, such as cleft lip/palate and heart malformations in children of women receiving phenytoin and other antiepileptic drugs, there have more recently been reports of a fetal hydantoin syndrome. This consists of prenatal growth deficiency, microcephaly, and mental deficiency in children born to mothers who have received phenytoin, barbiturates, alcohol, or trimethadione. However, these features are all interrelated and are frequently associated with intrauterine growth retardation from other causes.
There have been isolated reports of malignancies, including neuroblastoma, in children whose mothers received phenytoin during pregnancy.
An increase in seizure frequency during pregnancy occurs in a high proportion of patients, because of altered phenytoin absorption or metabolism. Periodic measurement of serum phenytoin levels is particularly valuable in the management of a pregnant epileptic patient as a guide to an appropriate adjustment of dosage. However, postpartum restoration of the original dosage will probably be indicated.
Neonatal coagulation defects have been reported within the first 24 hours in babies born to epileptic mothers receiving phenobarbital and/or phenytoin. Vitamin K has been shown to prevent or correct this defect and has been recommended to be given to the mother before delivery and the neonate after birth.
The addition of phenytoin sodium injection to intravenous infusion is not recommended due to lack of solubility and resultant precipitation.
Phenytoin sodium injection should be injected slowly (not exceeding 50 mg per minute in adults), directly into a large vein through a large gauge needle or intravenous catheter. Each injection of intravenous phenytoin sodium injection should be followed by an injection of sterile saline through the same needle or intravenous catheter to avoid local venous irritation due to the alkalinity of the solution. Continuous infusion should be avoided.
Soft tissue irritation and inflammation has occurred at the site of injection with and without extravasation of intravenous phenytoin. Soft tissue irritation may vary from slight tenderness to extensive necrosis, sloughing, and in rare instances has led to amputation. Improper administration including subcutaneous or perivascular injection should be avoided to help prevent possibility of the above.
Edema, discoloration and pain of the distal limb (described as “purple glove syndrome”) have been reported following peripheral intravenous phenytoin sodium injection. This may or may not be associated with extravasation. Although resolution of symptoms may be spontaneous, skin necrosis and limb ischemia have occurred and required such interventions as fasciotomies, skin grafting and amputation. Therefore, phenytoin sodium injection should be administered as described above.
The liver is the site of biotransformation. Patients with impaired liver function, elderly patients, or those who are gravely ill may show early toxicity.
A small percentage of individuals who have been treated with phenytoin have been shown to metabolize the drug slowly. Slow metabolism may be due to limited enzyme availability and lack of induction; it appears to be genetically determined.
Phenytoin should be discontinued if a skin rash appears (see WARNINGS section regarding drug discontinuation). If the rash is exfoliative, purpuric, or bullous or if lupus erythematosus or Stevens-Johnson syndrome is suspected, use of this drug should not be resumed and alternative therapy should be considered. (See
ADVERSE REACTIONS). If the rash is of a milder type (measles like or scarlatiniform), therapy may be resumed after the rash has completely disappeared. If the rash recurs upon reinstitution of therapy, further phenytoin medication is contraindicated.
Hyperglycemia, resulting from the drug’s inhibitory effects on insulin release, has been reported. Phenytoin may also raise the serum glucose level in diabetic patients.
Phenytoin is not indicated for seizures due to hypoglycemic or other metabolic causes. Appropriate diagnostic procedures should be performed as indicated.
Phenytoin is not effective for absence (petit mal) seizures. If tonic clonic (grand mal) and absence (petit mal) seizures are present, combined drug therapy is needed.
Serum levels of phenytoin sustained above the optimal range may produce confusional states referred to as “delirium”, “psychosis”, or “encephalopathy”, or rarely irreversible cerebellar dysfunction. Accordingly, at the first sign of acute toxicity, plasma levels are recommended. Dose reduction of phenytoin therapy is indicated if plasma levels are excessive; if symptoms persist, termination is recommended. (See WARNINGS)
Phenytoin serum level determinations may be necessary to achieve optimal dosage adjustments.
There are many drugs which may increase or decrease phenytoin levels or which phenytoin may affect. The most commonly occurring drug interactions are listed below:
1. Drugs which may increase phenytoin serum levels include: Chloramphenicol, dicumarol, disulfiram, tolbutamide, isoniazid, phenylbutazone, acute alcohol intake, salicylates, chlordiazepoxide, phenothiazines, diazepam, estrogens, ethosuximide, halothane, methylphenidate, sulfonamides, cimetidine, trazodone.
2. Drugs which may decrease phenytoin levels include: Carbamazepine, chronic alcohol abuse, reserpine. Moban® brand of molindone hydrochloride contains calcium ions which interfere with the absorption of phenytoin. Ingestion times of phenytoin and antacid preparations containing calcium should be staggered in patients with low serum phenytoin levels to prevent absorption problems.
3. Drugs which may either increase or decrease phenytoin serum levels include: Phenobarbital, valproic acid and sodium valproate. Similarly, the effect of phenytoin on phenobarbital, valproic acid and sodium valproate serum levels is unpredictable.
4. Although not a true drug interaction, tricyclic antidepressants may precipitate seizures in susceptible patients and phenytoin dosage may need to be adjusted.
5. Drugs whose efficacy is impaired by phenytoin include: Corticosteroids, coumarin anticoagulants, oral contraceptives, quinidine, vitamin D, digitoxin, rifampin, doxycycline, estrogens, furosemide. Serum level determinations are especially helpful when possible drug interactions are suspected.
Phenytoin may cause decreased serum levels of protein bound iodine (PBI). It may also produce lower than normal values for dexamethasone or metyrapone tests. Phenytoin may cause increased serum levels of glucose, alkaline phosphatase, and gamma glutamyl transpeptidase (GGT).
See WARNINGS section for information on carcinogenesis.
See WARNINGS .
Infant breast feeding is not recommended for women taking this drug because phenytoin appears to be secreted in low concentrations in human milk.
The most notable signs of toxicity associated with the intravenous use of this drug are cardiovascular collapse and/or central nervous system depression. Hypotension does occur when the drug is administered rapidly by the intravenous route. The rate of administration is very important; it should not exceed 50 mg per minute in adults, and 1 to 3 mg/ kg/ min in neonates. At this rate, toxicity should be minimized.
Cardiovascular
Severe cardiotoxic reactions and fatalities have been reported with atrial and ventricular conduction depression and ventricular fibrillation. Severe complications are most commonly encountered in elderly or gravely ill patients.
Central Nervous System
The most common manifestations encountered with phenytoin therapy are referable to this system and are usually dose related. These include nystagmus, ataxia, slurred speech, decreased coordination, and mental confusion. Dizziness, insomnia, transient nervousness, motor twitchings, and headaches have also been observed. There have also been rare reports of phenytoin induced dyskinesias, including chorea, dystonia, tremor, and asterixis, similar to those induced by phenothiazine and other neuroleptic drugs.
A predominantly sensory peripheral polyneuropathy has been observed in patients receiving long-term phenytoin therapy.
Gastrointestinal System
Nausea, vomiting, and constipation.
Integumentary System
Dermatological manifestations sometimes accompanied by fever have included scarlatiniform or morbilliform rashes. A morbilliform rash (measles like) is the most common; other types of dermatitis are seen more rarely. Other more serious forms which may be fatal have included bullous, exfoliative or purpuric dermatitis, lupus erythematosus, Stevens-Johnson syndrome, and toxic epidermal necrolysis (see PRECAUTIONS).
Hemopoietic System
Hemopoietic complications, some fatal, have occasionally been reported in association with administration of phenytoin. These have included thrombocytopenia, leukopenia, granulocytopenia, agranulocytosis, and pancytopenia with or without bone marrow suppression. While macrocytosis and megaloblastic anemia have occurred, these conditions usually respond to folic acid therapy. Lymphadenopathy including benign lymph node hyperplasia, pseudolymphoma, lymphoma, and Hodgkin’s Disease have been reported (see WARNINGS).
Connective Tissue System
Coarsening of the facial features, enlargement of the lips, gingival hyperplasia, hypertrichosis and Peyronie’s Disease.
Injection Site
Local irritation, inflammation, tenderness, necrosis and sloughing have been reported with or without extravasation of intravenous phenytoin.
Other
Systemic lupus erythematosus, periarteritis nodosa, toxic hepatitis, liver damage and immunoglobulin abnormalities and purple glove syndrome may occur. (See PRECAUTIONS.)
The lethal dose in children is not known. The lethal dose in adults is estimated to be 2 to 5 grams. The initial symptoms are nystagmus, ataxia, and dysarthria. Other signs are tremor, hyperflexia, lethargy, slurred speech, nausea, vomiting. The patient may become comatose and hypertensive. Death is due to respiratory and circulatory depression.
There are marked variations among individuals with respect to phenytoin plasma levels where toxicity may occur.
Nystagmus, on lateral gaze usually appears at 20 mcg/mL, ataxia at 30 mcg/mL, dysarthria and lethargy appear when the plasma concentration is over 40 mcg/mL, but as high a concentration as 50 mcg/mL has been reported without evidence of toxicity. As much as 25 times the therapeutic dose has been taken to result in a serum concentration over 100 mcg/mL with complete recovery.
Treatment
Treatment is nonspecific since there is no known antidote.
The adequacy of the respiratory and circulatory systems should be carefully observed, and appropriate supportive measures employed. Hemodialysis can be considered since phenytoin is not completely bound to plasma proteins.
Total exchange transfusion has been used in the treatment of severe intoxication in pediatric patients.
In acute overdosage the possibility of other CNS depressants, including alcohol, should be borne in mind.
The addition of phenytoin sodium injection to intravenous infusion is not recommended due to lack of solubility and resultant precipitation.
Not to exceed 50 mg per minute, intravenously in adults, and not exceeding 1 to 3 mg/kg/min in neonates.
There is a relatively small margin between full therapeutic effect and minimally toxic doses of this drug.
The solution is suitable for use as long as it remains free of haziness and precipitate. Upon refrigeration or freezing, a precipitate might form; this will dissolve again after the solution is allowed to stand at room temperature. The product is still suitable for use. Only a clear solution should be used. A faint yellow coloration may develop; however, this has no effect on the potency of the solution.
In the treatment of status epilepticus, the intravenous route is preferred because of the delay in absorption of phenytoin when administered intramuscularly.
Serum concentrations should be monitored and care should be taken when switching a patient from the sodium salt to the free acid form.
Phenytoin sodium injection is formulated with the sodium salt of phenytoin. Because there is approximately an 8% increase in drug content with the free acid form over that of the sodium salt, dosage adjustments and serum level monitoring may be necessary when switching from a product formulated with the free acid to a product formulated with the sodium salt and vice versa.
Status Epilepticus
In adults, a loading dose of 10 to 15 mg/kg should be administered slowly intravenously, at a rate not exceeding 50 mg per minute (this will require approximately 20 minutes in a 70 kg patient). The loading dose should be followed by maintenance doses of 100 mg orally or intravenously every 6 to 8 hours.
Recent work in neonates and pediatric patients has shown that absorption of phenytoin is unreliable after oral administration, but a loading dose of 15 to 20 mg/kg of phenytoin intravenously will usually produce plasma concentrations of phenytoin within the generally accepted therapeutic range (10 to 20 mcg/mL). The drug should be injected slowly intravenously at a rate not exceeding 1 to 3 mg/kg/min.
Phenytoin sodium injection should be injected slowly and directly into a large vein through a large gauge needle or intravenous catheter. Each injection of intravenous phenytoin should be followed by an injection of sterile saline through the same needle or catheter to avoid local venous irritation due to alkalinity of the solution. Continuous infusion should be avoided; the addition of phenytoin sodium injection to intravenous infusion fluids is not recommended because of the likelihood of precipitation.
Continuous monitoring of the electrocardiogram and blood pressure is essential. The patient should be observed for signs of respiratory depression. Determination of phenytoin plasma levels is advised when using phenytoin in the management of status epilepticus and in the subsequent establishment of maintenance dosage.
Other measures, including concomitant administration of an intravenous benzodiazepine such as diazepam, or an intravenous short acting barbiturate, will usually be necessary for rapid control of seizures because of the required slow rate of administration of phenytoin.
If administration of phenytoin sodium injection does not terminate seizures, the use of other anticonvulsants, intravenous barbiturates, general anesthesia, and other appropriate measures should be considered.
Intramuscular administration should not be used in the treatment of status epilepticus because the attainment of peak plasma levels may require up to 24 hours.
Neurosurgery
Prophylactic dosage is 100 to 200 mg (2 to 4 mL) intramuscularly at approximately 4 hour intervals during surgery and continued during the postoperative period. When intramuscular administration is required for a patient previously stabilized orally, compensating dosage adjustments are necessary to maintain therapeutic plasma levels. An intramuscular dose 50% greater than the oral dose is necessary to maintain these levels. When returned to oral administration, the dose should be reduced by 50% of the original oral dose for one week to prevent excessive plasma levels due to sustained release from intramuscular tissue sites.
If the patient requires more than a week of IM phenytoin, alternative routes should be explored, such as gastric intubation. For time periods less than one week, the patient shifted back from IM administration should receive one half the original oral dose for the same period of time the patient received IM phenytoin. Monitoring plasma levels would help prevent a fall into the subtherapeutic range. Serum blood level determinations are especially helpful when possible drug interactions are suspected.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Phenytoin Sodium Injection, USP 100 mg/2 mL (50 mg/mL) in a 2 mL vial (NDC 39822-4050-2) packaged in carton of 25 (NDC 39822-4050-3)
Phenytoin Sodium Injection, USP 250 mg/5 mL (50 mg/mL) in a 10 mL vial (NDC 39822-4050-5) packaged in carton of 25 (NDC 39822-4050-6)
Store at 20° to 25°C (68º to 77°F) [see USP Controlled Room Temperature].
Manufactured by: HIKMA FARMACEUTICA (PORTUGAL), S.A.
Estrada do Rio da Mó, 8, 8A e 8B – Fervença
2705-906 Terrugem SNT, PORTUGAL
Distributed by: X-GEN Pharmaceuticals
Big Flats, NY 14814
Revised: 06/2011
Phenytoin Sodium Injection,
USP 250 mg/5mL (50 mg/mL)
1 x 5 mL Single Dose Vial

PHENYTOIN SODIUMPHENYTOIN SODIUM INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||