Phenylephrine Hydrochloride
General Injectables & Vaccines, Inc
Phenylephrine Hydrochloride 1% 10mg/mL SDV
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- PHENYLEPHRINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PHENYLEPHRINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- PHENYLEPHRINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL
FULL PRESCRIBING INFORMATION
BOXED WARNING
Rx Only
CONTAINS NO ANTIMICROBIAL PRESERVATIVE
WARNING
PHYSICIANS SHOULD COMPLETELY FAMILIARIZE THEMSELVES WITH THE COMPLETE CONTENTS OF THIS
INSERT BEFORE PRESCRIBING PHENYLEPHRINE HYDROCHLORIDE INJECTION, USP.
PHENYLEPHRINE HYDROCHLORIDE DESCRIPTION
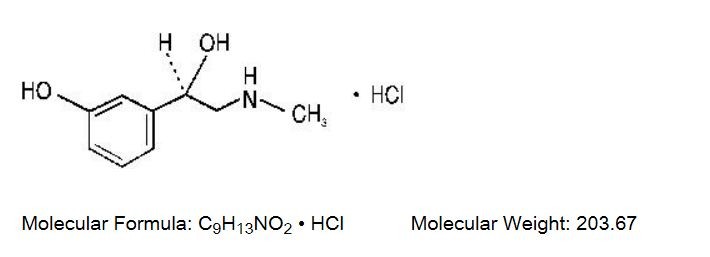
Phenylephrine Hydrochloride, a synthetic sympathomimetic agent, is a vasoconstrictor and pressor drug chemically related to epinephrine and ephedrine that is available as a sterile solution for parenteral injection. Each mL contains: phenylephrine HCI 10 mg, sodium metabisulfite 2 mg, sodium chloride 3.5 mg, sodium citrate (dihydrate) 4 mg, citric acid (anhydrous) 0.914 mg, water for injection q.s. pH (between 3.0 and 6.5) adjusted with citric acid and/or sodium hydroxide. Chemically, it is (-) - m - Hydroxy - a - [(methylamino)methyl] benzyl alcohol hydrochloride. The structural formula is as follows:
CLINICAL PHARMACOLOGY
INDICATIONS & USAGE
PHENYLEPHRINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
Phenylephrine hydrochloride should be employed only with extreme caution in elderly patients or in patients with hyperthyroidism, bradycardia, partial heart block, myocardial disease, or severe arteriosclerosis.
PHENYLEPHRINE HYDROCHLORIDE ADVERSE REACTIONS
Headache, reflex bradycardia, excitability, restlessness, and rarely arrhythmias.
OVERDOSAGE
DOSAGE & ADMINISTRATION
Phenylephrine hydrochloride is generally injected subcutaneously, intramuscularly, slowly intravenously, or in dilute solution as a continuous intravenous infusion. In patients with paroxysmal supraventricular tachycardia and, if indicated, in case of emergency, phenylephrine hydrochloride is administered directly intravenously. The dose should be adjusted according to the pressor response.
| Dosage Calculations |
Use |
| Dose Required |
Phenylephrine HCl Injection 1% |
| 10 mg |
1 mL |
| 5 mg |
0.5 mL |
| 1 mg |
0.1 mL |
|
|
|
|
|
|
|
|
Use Diluted |
| Dose Required |
Phenylephrine HCI Injection 0.1% |
| 0.1 mg |
0.1 mL |
| 0.2 mg |
0.2 mL |
| 0.5 mg |
0.5 mL |
HOW SUPPLIED
PACKAGE LABEL

Phenylephrine HydrochloridePhenylephrine Hydrochloride INJECTION, SOLUTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||