Perphenazine and Amitriptyline Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- DRUG INTERACTIONS
- PEDIATRIC USE
- PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL MEDGUIDE
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNING
Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of seventeen placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in drug-treated patients of between 1.6 to 1.7 times the risk of death in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group. Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. Observational studies suggest that, similar to atypical antipsychotic drugs, treatment with conventional antipsychotic drugs may increase mortality. The extent to which the findings of increased mortality in observational studies may be attributed to the antipsychotic drug as opposed to some characteristic(s) of the patients is not clear. Perphenazine and amitriptyline hydrochloride is not approved for the treatment of patients with dementia-related psychosis (seeWARNINGS).
Suicidality and Antidepressant Drugs
WARNINGS: Clinical Worsening and Suicide Risk,PRECAUTIONS: Information for PatientsandPRECAUTIONS: Pediatric Use.)
PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE DESCRIPTION
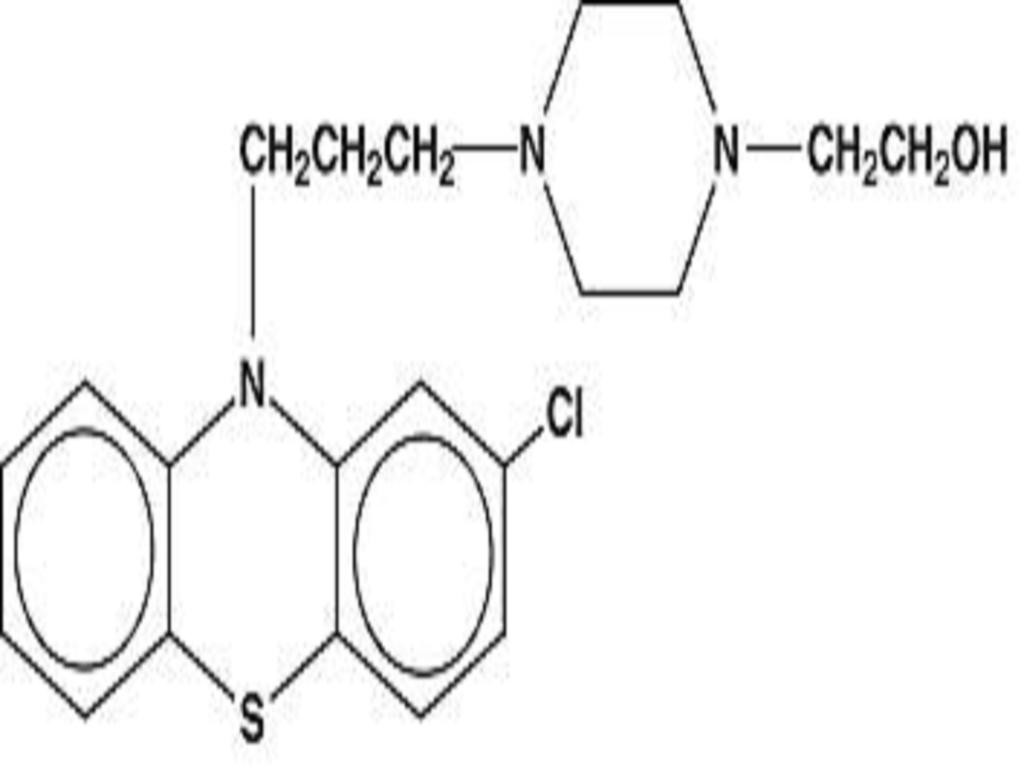
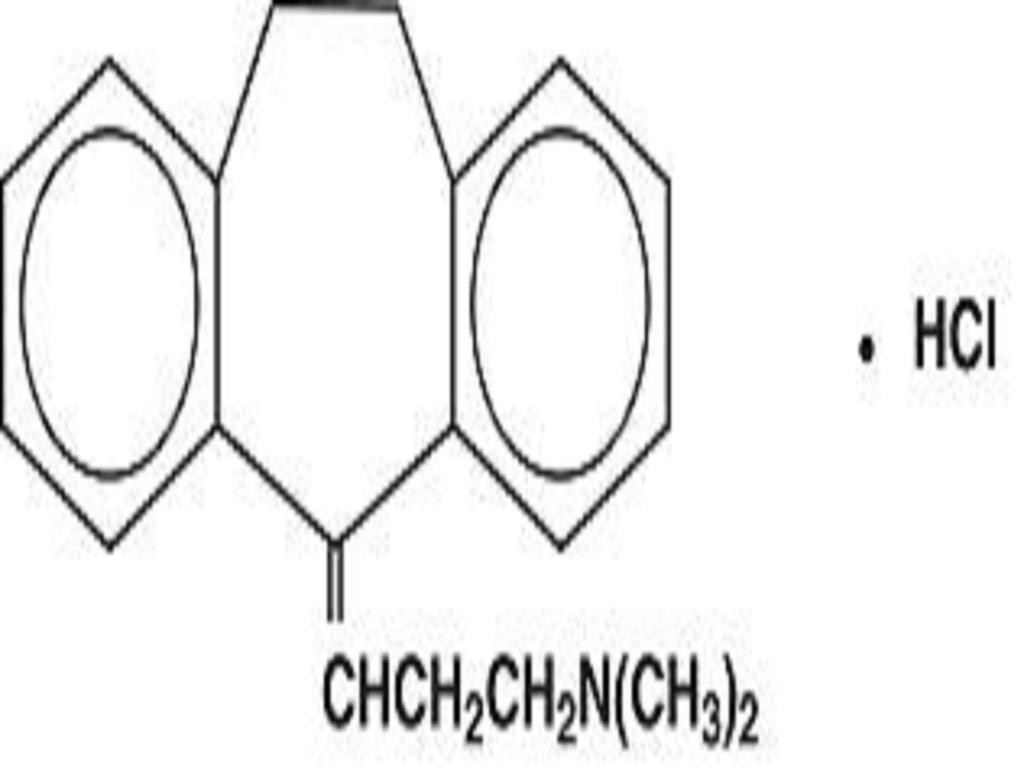
CLINICAL PHARMACOLOGY
PerphenazineAmitriptyline Hydrochloride
INDICATIONS & USAGE
PERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
Increased Mortality in Elderly Patients with Dementia-Related PsychosisElderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Perphenazine and amitriptyline hydrochloride is not approved for the treatment of patients with dementia-related psychosis (seeBOXED WARNING).
Clinical Worsening and Suicide Risk
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Screening Patients for Bipolar Disorder
Tardive Dyskinesia
ADVERSE REACTIONS
Neuroleptic Malignant Syndrome (NMS)
General
Usage in Pregnancy
Pregnancy
Nonteratogenic Effects
PRECAUTIONS
GeneralPerphenazine
Amitriptyline Hydrochloride
INFORMATION FOR PATIENTS
Clinical Worsening and Suicide Risk
DRUG INTERACTIONS
Drugs Metabolized by P450 2D6
Perphenazine
Amitriptyline Hydrochloride
PEDIATRIC USE
BOX WARNINGWARNINGS: Clinical Worsening and Suicide RiskPERPHENAZINE AND AMITRIPTYLINE HYDROCHLORIDE ADVERSE REACTIONS
Perphenazine
WARNINGS
Neurological
Tardive Dyskinesia
Cardiovascular
CNS and Neuromuscular
Neurological
Autonomic:
Allergic:
Hematologic:
Gastrointestinal:
Dermatologic:
Ophthalmic:
Endocrine:
Other:
CNS and Neuromuscular:
Gastrointestinal:
Dermatologic:
Ophthalmic:
Endocrine:
Amitriptyline Hydrochloride
Cardiovascular:
CNS and Neuromuscular:
Anticholinergic:
Allergic:
Hematologic:
Gastrointestinal:
Endocrine:
Other:
Withdrawal Symptoms
OVERDOSAGE
Manifestations
ADVERSE REACTIONS
Critical manifestations of tricyclic antidepressant overdose includes: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression, including coma. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of tricyclic antidepressant toxicity. Other signs of overdose may include: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia, or any of the symptoms listed underADVERSE REACTIONS.
Management
General
Gastrointestinal Decontamination
All patients suspected of tricyclic antidepressant overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular
A maximal limb lead QRS duration of0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed) should be instituted for patients with dysrhythmias and/or QRS widening. A pH > 7.60 or a pCO2 < 20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in tricyclic antidepressant poisoning.
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
Psychiatric Follow-Up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of child and adult overdosages are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
DOSAGE & ADMINISTRATION
Initial Dosage
In more severely ill patients with schizophrenia
Maintenance Dosage
HOW SUPPLIED
STORAGE AND HANDLING
SPL MEDGUIDE
Talk to your, or your family member's, healthcare provider about:
-
● all risks and benefits of treatment with antidepressant medicines
-
● all treatment choices for depression or other serious mental illness
1. Antidepressant medicines may increase suicidal thoughts or actions in some children, teenagers, and young adults within the first few months of treatment.
2. Depression and other serious mental illnesses are the most important causes of suicidal thoughts and actions. Some people may have a particularly high risk of having suicidal thoughts or actions.
3. How can I watch for and try to prevent suicidal thoughts and actions in myself or a family member?
-
● Pay close attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings. This is very important when an antidepressant medicine is started or when the dose is changed.
-
● Call the healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.
-
● Keep all follow-up visits with the healthcare provider as scheduled. Call the healthcare provider between visits as needed, especially if you have concerns about symptoms.
-
● thoughts about suicide or dying
-
● attempts to commit suicide
-
● new or worse depression
-
● new or worse anxiety
-
● feeling very agitated or restless
-
● panic attacks
-
● trouble sleeping (insomnia)
-
● new or worse irritability
-
● acting aggressive, being angry, or violent
-
● acting on dangerous impulses
-
● an extreme increase in activity and
-
● other unusual changes in behavior or
What else do I need to know about antidepressant medicines?
-
● Never stop an antidepressant medicine without first talking to a healthcare provider. Stopping an antidepressant medicine suddenly can cause other symptoms.
-
● Antidepressants are medicines used to treat depression and other illnesses.It is important to discuss all the risks of treating depression and also the risks of not treating it. Patients and their families or other caregivers should discuss all treatment choices with the healthcare provider, not just the use of antidepressants.
-
● Antidepressant medicines have other side effects.Talk to the healthcare provider about the side effects of the medicine prescribed for you or your family member.
-
● Antidepressant medicines can interact with other medicines.Know all of the medicines that you or your family member takes. Keep a list of all medicines to show the healthcare provider. Do not start new medicines without first checking with your healthcare provider.
-
● Not all antidepressant medicines prescribed for children are FDA approved for use in children.Talk to your child's healthcare provider for more information.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Perphenazine and Amitriptyline HydrochloridePerphenazine and Amitriptyline Hydrochloride TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!