oxycodone hydrochloride and ibuprofen
Oxycodone Hydrochloride and Ibuprofen Tablets CII Revised: July 2008 Rx only 173189
FULL PRESCRIBING INFORMATION: CONTENTS*
- OXYCODONE HYDROCHLORIDE AND IBUPROFEN DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- OXYCODONE HYDROCHLORIDE AND IBUPROFEN INDICATIONS AND USAGE
- OXYCODONE HYDROCHLORIDE AND IBUPROFEN CONTRAINDICATIONS
- WARNINGS
- Cardiovascular Effects
- Hypertension
- Congestive Heart Failure and Edema
- Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
- Misuse Abuse and Diversion of Opioids
- Respiratory Depression
- Hypotensive Effect
- Head Injury and Increased Intracranial Pressure
- Acute Abdominal Conditions
- Anaphylactoid Reactions
- Renal Effects
- Advanced Renal Disease
- Skin Reactions
- Pregnancy
- Interactions with Alcohol and Drugs of Abuse
- PRECAUTIONS
- General
- Special Risk Patients
- Use in Pancreatic/Biliary Tract Disease
- Cough Reflex
- Hepatic Effects
- Hematological Effects
- Pre-existing Asthma
- Aseptic Meningitis
- Information for Patients
- Laboratory Tests
- Drug Interactions
- Carcinogenesis, Mutagenesis and Impairment of Fertility
- Pregnancy
- Labor and Delivery
- Nursing Mothers
- Pediatric Use
- Geriatric Use
- OXYCODONE HYDROCHLORIDE AND IBUPROFEN ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- OXYCODONE HYDROCHLORIDE AND IBUPROFEN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Cardiovascular Risk
-
NSAIDs may cause an increased risk of serious cardiovascular thrombotic events, myocardial infarction, and stroke, which can be fatal. This risk may increase with duration of use. Patients with cardiovascular disease or risk factors for cardiovascular disease may be at greater risk (See WARNINGS ).
-
Oxycodone hydrochloride and ibuprofen tablets are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).
Gastrointestinal Risk
-
NSAIDs cause an increased risk of serious gastrointestinal adverse events including bleeding, ulceration, and perforation of the stomach or intestines, which can be fatal. These events can occur at any time during use and without warning symptoms. Elderly patients are at greater risk for serious gastrointestinal events (See WARNINGS ).
OXYCODONE HYDROCHLORIDE AND IBUPROFEN DESCRIPTION
Each combination tablet contains:
Oxycodone hydrochloride, USP 5 mg
Ibuprofen, 400 mg.
Oxycodone Hydrochloride and Ibuprofen Tablets, 5 mg/400 mg are supplied in a fixed combination tablet form for oral administration and combine the opioid analgesic agent, oxycodone hydrochloride, with the nonsteroidal anti-inflammatory (NSAID) agent, ibuprofen.
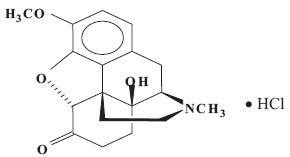
Oxycodone hydrochloride is a centrally acting semisynthetic opioid analgesic. Its chemical name is 4,5α-Epoxy-14-hydroxy-3-methoxy-methylmorphinan-6-one hydrochloride. Its molecular formula is C18H21NO4.HCl and molecular weight is 351.83. Its structural formula is:
Inactive ingredients in oxycodone hydrochloride and ibuprofen tablets include: sodium starch glycolate, microcrystalline cellulose, colloidal silicon dioxide, stearic acid, carboxymethylcellulose sodium, lactose monohydrate and talc. The film coating for the tablets include: Opadry® White II, Y-22 - 7719 and Opadry® Clear YS-1-19025-A, which consist of titanium dioxide, polydextrose, hypromellose, triacetin, polyethylene glycol 400 and polyethylene glycol 8000.
CLINICAL PHARMACOLOGY
Oxycodone hydrochloride component:
Oxycodone hydrochloride is a semisynthetic opioid analgesic with multiple actions which involve the central nervous system and smooth muscle. The mechanism of action of oxycodone is not known but is thought to be related to its binding to opiate receptors in the central nervous system. In addition to analgesia, opioids may produce sedation and respiratory depression.
Ibuprofen component:
Ibuprofen is a nonsteroidal anti-inflammatory agent that possesses analgesic and antipyretic activities. Its mode of action, similar to other NSAIDs, is not completely understood, but is thought to be related to its inhibition of cyclooxygenase activity and prostaglandin synthesis. Ibuprofen is a peripherally acting analgesic. Ibuprofen does not have any known effects on opiate receptors.
Pharmacokinetics
Absorption:
Oxycodone is rapidly absorbed after single dose administration of oxycodone hydrochloride and ibuprofen tablets. Maximum concentrations (Cmax) of oxycodone, ranging from 9.8 ng/mL to 11.7 ng/mL, are obtained within 1.3 hr to 2.1 hr after administration of oxycodone hydrochloride and ibuprofen tablets. Repeated administration of oxycodone hydrochloride and ibuprofen tablets every 6 hours results in approximately 50-65% increase in Cmax. In the presence of food, the bioavailability of oxycodone is slightly (25%) increased.
Ibuprofen is rapidly absorbed after oral administration of oxycodone hydrochloride and ibuprofen tablets. Cmax values range from 18.5 mcg/mL to 34.3 mcg/mL and are reached 1.6 hr to 3.1 hr after oral administration of oxycodone hydrochloride and ibuprofen tablets. Repeated administration of oxycodone hydrochloride and ibuprofen tablets every 6 hours does not result in any accumulation of ibuprofen. The bioavailability of ibuprofen is not altered in the presence of food.
Distribution:
Oxycodone binding to protein in serum is approximately 45%.
Ibuprofen is extensively bound to plasma proteins (99%).
Metabolism:
Oxycodone is metabolized in the liver by means of N-demethylation and O-demethylation, 6-ketoreduction and glucuronidation. The major circulating metabolite is noroxycodone, which possesses weak analgesic activity.
Oxymorphone, the end product of O-demethylation, has analgesic activity but is present in the plasma at low concentrations. Metabolism of oxycodone to oxymorphone occurs via CYP2D6.
Ibuprofen is present as a racemate and following absorption, it undergoes interconversion in the plasma from the R-isomer to the S-isomer.
Both the R- and S- isomers are metabolized to two primary metabolites: (+)-2-4’-(2-hydroxy-2-methyl-propyl) phenyl propionic acid and (+)-2-4’-(2-carboxypropyl) phenyl propionic acid, both of which circulate in the plasma at low levels relative to the parent.
Elimination:
Oxycodone is eliminated from the systemic circulation with half life (T1/2) values ranging from 3.1 hr to 3.7 hr after single dose administration of oxycodone hydrochloride and ibuprofen tablets. Urinary excretion of unchanged oxycodone amounts to approximately 4% of the administered oxycodone dose.
Ibuprofen is eliminated from the systemic circulation with half life (T1/2) values ranging from 1.8 hr to 2.6 hr after single dose administration of oxycodone hydrochloride and ibuprofen tablets. Urinary excretion of unchanged ibuprofen is minimal (less than 0.2% of administered ibuprofen dose).
Special Populations:
Gender: There are no gender effects on the pharmacokinetics of oxycodone or ibuprofen after administration of oxycodone hydrochloride and ibuprofen tablets.
Age: The effects of age on the pharmacokinetics of oxycodone hydrochloride and ibuprofen after administration of oxycodone hydrochloride and ibuprofen tablets have not been evaluated.
When either drug was administered alone, the pharmacokinetics of oxycodone and ibuprofen were similar in elderly subjects, compared to young healthy subjects.
Pediatrics: The pharmacokinetics of oxycodone and ibuprofen after administration of oxycodone hydrochloride and ibuprofen tablets have not been evaluated in a pediatric population.
Renal Impairment: The effects of renal impairment on the pharmacokinetics of oxycodone and ibuprofen after administration of oxycodone hydrochloride and ibuprofen tablets have not been evaluated.
Hepatic Impairment: The effects of hepatic impairment on the pharmacokinetics of oxycodone and ibuprofen after administration of oxycodone hydrochloride and ibuprofen tablets have not been evaluated. (See PRECAUTIONS; Hepatic Effects )
CLINICAL STUDIES
Oxycodone hydrochloride and ibuprofen tablets were investigated in three clinical studies. Two studies involving a total of 949 patients following dental surgery (removal of ipsilateral molars) and a third study of 456 patients following abdominal/pelvic surgery were conducted. In the three studies patients were administered a single dose of oxycodone hydrochloride and ibuprofen tablets, ibuprofen alone, oxycodone hydrochloride alone or placebo for acute, moderate to severe pain.
In these single dose studies, oxycodone hydrochloride and ibuprofen tablets produced greater efficacy than placebo and each of oxycodone hydrochloride and ibuprofen tablet’s individual components as measured by the magnitude of pain relief and the reduction in pain intensity through six hours. No multiple dose efficacy studies have been performed with oxycodone hydrochloride and ibuprofen tablets.
OXYCODONE HYDROCHLORIDE AND IBUPROFEN INDICATIONS AND USAGE
Carefully consider the potential benefits and risks of oxycodone hydrochloride and ibuprofen tablets and other treatment options before deciding to use oxycodone hydrochloride and ibuprofen tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ).
Oxycodone hydrochloride and ibuprofen tablets are indicated for the short term (no more than 7 days) management of acute, moderate to severe pain.
OXYCODONE HYDROCHLORIDE AND IBUPROFEN CONTRAINDICATIONS
Oxycodone hydrochloride and ibuprofen tablets should not be administered to patients who have previously exhibited hypersensitivity to oxycodone hydrochloride, ibuprofen, or any of oxycodone hydrochloride and ibuprofen tablet’s components.
Oxycodone hydrochloride and ibuprofen tablets should not be administered in any situation where opioids are contraindicated. This includes patients with significant respiratory depression (in unmonitored settings or the absence of resuscitative equipment) and patients with acute or severe bronchial asthma or hypercarbia. Patients known to be hypersensitive to other opioids may exhibit cross-sensitivity to oxycodone. Oxycodone hydrochloride and ibuprofen tablets are contraindicated in any patient who has or is suspected of having paralytic ileus.
Oxycodone hydrochloride and ibuprofen tablets should not be given to patients who have experienced asthma, urticaria, or allergic-type reactions after taking aspirin or other NSAIDs. Severe anaphylactoid reactions to NSAIDs, some of which were fatal, have been reported in such patients (see WARNINGS; Anaphylactoid Reactions , and PRECAUTIONS; Pre-existing Asthma ).
Oxycodone hydrochloride and ibuprofen tablets are contraindicated for the treatment of peri-operative pain in the setting of coronary artery bypass graft (CABG) surgery (see WARNINGS ).
WARNINGS
Cardiovascular Effects
Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, myocardial infarction, and stroke, which can be fatal. All NSAIDs, both COX-2 selective and nonselective, may have a similar risk. Patients with known CV disease or risk factors for CV disease may be at greater risk. To minimize the potential risk for an adverse CV event in patients treated with an NSAID, the lowest effective dose should be used for the shortest duration possible. Physicians and patients should remain alert for the development of such events, even in the absence of previous CV symptoms. Patients should be informed about the signs and/or symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID does increase the risk of serious GI events (see WARNINGS; Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation ).
Two large, controlled, clinical trials of a COX-2 selective NSAID for the treatment of pain in the first 10-14 days following CABG surgery found an increased incidence of myocardial infarction and stroke (see CONTRAINDICATIONS).
Hypertension
NSAIDs, including oxycodone hydrochloride and ibuprofen tablets, can lead to onset of new hypertension or worsening of pre-existing hypertension, either of which may contribute to the increased incidence of CV events. Patients taking thiazides or loop diuretics may have impaired response to these therapies when taking NSAIDs. NSAIDs, including oxycodone hydrochloride and ibuprofen, should be used with caution in patients with hypertension. Blood pressure (BP) should be monitored closely during the initiation of NSAID treatment and throughout the course of therapy.
Congestive Heart Failure and Edema
Fluid retention and edema have been observed in some patients taking NSAIDs. Oxycodone hydrochloride and ibuprofen tablets should be used with caution in patients with fluid retention or heart failure.
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
NSAIDs, including oxycodone hydrochloride and ibuprofen tablets, can cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients, who develop a serious upper GI adverse event on NSAID therapy, is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occur in approximately 1% of patients treated for 3-6 months, and in about 2-4% of patients treated for one year. These trends continue with longer duration of use, increasing the likelihood of developing a serious GI event at some time during the course of therapy. However, even short-term therapy is not without risk.
NSAIDs should be prescribed with extreme caution in those with a prior history of ulcer disease or gastrointestinal bleeding. Patients with a prior history of peptic ulcer disease and/or gastrointestinal bleeding who use NSAIDs have a greater than 10-fold increased risk for developing a GI bleed compared to patients with neither of these risk factors. Other factors that increase risk for GI bleeding in patients treated with NSAIDs include concomitant use of oral corticosteroids or anticoagulants, longer duration of NSAID therapy, smoking, use of alcohol, older age, and poor general health status. Most spontaneous reports of fatal GI events are in elderly or debilitated patients and therefore, special care should be taken in treating this population.
To minimize the potential risk for an adverse GI event in patients treated with an NSAID, the lowest effective dose should be used for the shortest possible duration. Patients and physicians should remain alert for signs and symptoms of GI ulceration and bleeding during NSAID therapy and promptly initiate additional evaluation and treatment if a serious GI adverse event is suspected. This should include discontinuation of the NSAID until a serious GI adverse event is ruled out. For high risk patients, alternate therapies that do not involve NSAIDs should be considered.
Misuse Abuse and Diversion of Opioids
Oxycodone hydrochloride and ibuprofen tablets contain oxycodone, which is an opioid agonist, and a Schedule II controlled substance. Opioid agonists have the potential for being abused and are sought by abusers and people with addiction disorders, and are subject to diversion.
Oxycodone hydrochloride and ibuprofen tablets can be abused in a manner similar to other opioid agonists, legal or illicit. This should be considered when prescribing or dispensing oxycodone hydrochloride and ibuprofen tablets in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse or diversion (see DRUG ABUSE AND DEPENDENCE ).
Respiratory Depression
Oxycodone may produce dose-related respiratory depression by acting directly on the brain stem respiratory centers. Oxycodone hydrochloride also affects the center that controls respiratory rhythm, and may produce irregular and periodic breathing. Respiratory depression occurs most frequently in elderly or debilitated patients, usually following large initial doses in non-tolerant patients, or when opioids are given in conjunction with other agents that depress respiration. Oxycodone hydrochloride and ibuprofen tablets should be used with extreme caution in patients with significant chronic obstructive pulmonary disease or cor pulmonale, and in patients having substantially decreased respiratory reserve, hypoxia, hypercapnia, or pre-existing respiratory depression. In such patients, even usual therapeutic doses of oxycodone hydrochloride and ibuprofen tablets may decrease respiratory drive to the point of apnea.
Hypotensive Effect
Oxycodone hydrochloride and ibuprofen tablets, like all opioid analgesics, may cause severe hypotension in an individual whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents which compromise vasomotor tone. Oxycodone hydrochloride and ibuprofen tablets may produce orthostatic hypotension in ambulatory patients. Oxycodone hydrochloride and ibuprofen tablets, like all opioid analgesics, should be administered with caution to patients in circulatory shock, since vasodilatation produced by the drug may further reduce cardiac output and blood pressure.
Head Injury and Increased Intracranial Pressure
The respiratory depressant effects of opioids and their capacity to elevate cerebrospinal fluid pressure may be markedly exaggerated in the presence of head injury, intracranial lesions or a pre-existing increase in intracranial pressure. Furthermore, opioids produce adverse reactions that may obscure the clinical course of patients with head injuries.
Acute Abdominal Conditions
The administration of opioids may obscure the diagnosis or clinical course of patients with acute abdominal conditions.
Anaphylactoid Reactions
Anaphylactoid reactions may occur in patients without known prior exposure to oxycodone hydrochloride and ibuprofen tablets. Oxycodone hydrochloride and ibuprofen tablets should not be given to patients with the aspirin triad or a history of angioedema. The triad typically occurs in asthmatic patients who experience rhinitis with or without nasal polyps, or who exhibit severe, potentially fatal bronchospasm after taking aspirin or other NSAIDs. Fatal reactions to NSAIDs have been reported in such patients (see CONTRAINDICATIONS and PRECAUTIONS; Pre-existing Asthma ). Emergency help should be sought when anaphylactoid reaction occurs.
Renal Effects
Long-term administration of NSAIDs has resulted in renal papillary necrosis and other renal injury. Renal toxicity has also been seen in patients in whom renal prostaglandins have a compensatory role in the maintenance of renal perfusion. In these patients, administration of a nonsteroidal anti-inflammatory drug may cause a dose-dependent reduction in prostaglandin formation and, secondarily, in renal blood flow, which may precipitate overt renal decompensation. Patients at greatest risk of this reaction are those with impaired renal function, heart failure, liver dysfunction, those taking diuretics and ACE inhibitors, and the elderly. Discontinuation of NSAID therapy is usually followed by recovery to the pretreatment state.
Advanced Renal Disease
In patients with advanced kidney disease, treatment with oxycodone hydrochloride and ibuprofen tablets is not recommended. No information is available from controlled clinical studies regarding the use of oxycodone hydrochloride and ibuprofen in patients with advanced renal disease. However, if oxycodone hydrochloride and ibuprofen tablets therapy must be initiated, due to the NSAID component, close monitoring of the patient’s kidney function is advisable (see WARNINGS; Renal Effects ).
Skin Reactions
NSAIDs, including oxycodone hydrochloride and ibuprofen tablets, can cause serious skin adverse events such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Patients should be informed about the signs and symptoms of serious skin manifestations and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
Pregnancy
As with other NSAID-containing products, oxycodone hydrochloride and ibuprofen tablets should be avoided in late pregnancy because it may cause premature closure of the ductus arteriosus.
Interactions with Alcohol and Drugs of Abuse
Oxycodone may be expected to have additive effects when used in conjunction with alcohol, other opioids, or illicit drugs that cause central nervous system depression.
PRECAUTIONS
General
Oxycodone hydrochloride and ibuprofen tablets cannot be expected to substitute for corticosteroids or to treat corticosteroid insufficiency. Abrupt discontinuation of corticosteroids may lead to disease exacerbation. Patients on prolonged corticosteroid therapy should have their therapy tapered slowly if a decision is made to discontinue corticosteroids.
The pharmacological activity of oxycodone hydrochloride and ibuprofen tablets in reducing fever and inflammation may diminish the utility of these diagnostic signs in detecting complications of presumed noninfectious, painful conditions.
Special Risk Patients
As with any opioid analgesic agent, oxycodone hydrochloride and ibuprofen tablets should be used with caution in elderly or debilitated patients, and those with severe impairment of hepatic, pulmonary or renal function, hypothyroidism, Addison’s disease, acute alcoholism, convulsive disorders, CNS depression or coma, delirium tremens, kyphoscoliosis associated with respiratory depression, toxic psychosis, prostatic hypertrophy or urethral stricture. The usual precautions should be observed and the possibility of respiratory depression, postural hypotension, and altered mental states should be kept in mind.
Use in Pancreatic/Biliary Tract Disease
Oxycodone hydrochloride and ibuprofen tablets may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis. Opioids like oxycodone hydrochloride and ibuprofen tablets may cause increases in the serum amylase level.
Cough Reflex
Oxycodone suppresses the cough reflex; as with other opioid containing products, caution should be exercised when oxycodone hydrochloride and ibuprofen tablets are used postoperatively and in patients with pulmonary disease.
Hepatic Effects
Borderline elevations of one or more liver tests may occur in up to 15% of patients taking NSAIDs including ibuprofen as found in oxycodone hydrochloride and ibuprofen tablets. These laboratory abnormalities may progress, may remain unchanged, or may be transient with continuing therapy. Notable elevations of ALT or AST (approximately three or more times the upper limit of normal) have been reported in approximately 1% of patients in clinical trials with NSAIDs. In addition, rare cases of severe hepatic reactions, including jaundice and fatal fulminant hepatitis, liver necrosis and hepatic failure, some of them with fatal outcomes have been reported.
A patient with symptoms and/or signs suggesting liver dysfunction, or in whom an abnormal liver test has occurred, should be evaluated for evidence of the development of a more severe hepatic reaction while on therapy with oxycodone hydrochloride and ibuprofen tablets. If clinical signs and symptoms consistent with liver disease develop, or if systematic manifestations occur (e.g., eosinophilia, rash, etc.), oxycodone hydrochloride and ibuprofen tablets should be discontinued.
Hematological Effects
Anemia is sometimes seen in patients receiving NSAIDs, including ibuprofen as found in oxycodone hydrochloride and ibuprofen tablets. This may be due to fluid retention, occult or gross GI blood loss, or an incompletely described effect upon erythropoiesis. Patients on long-term treatment with NSAIDs, including ibuprofen, should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
NSAIDs inhibit platelet aggregation and have been shown to prolong bleeding time in some patients. Unlike aspirin, their effect on platelet function is quantitatively less, of shorter duration, and reversible. Patients receiving oxycodone hydrochloride and ibuprofen tablets who may be adversely affected by alterations in platelet function, such as those with coagulation disorders or patients receiving anticoagulants, should be carefully monitored. Patients previously treated with NSAIDs and currently using oxycodone hydrochloride and ibuprofen tablets should have their hemoglobin or hematocrit checked if they exhibit any signs or symptoms of anemia.
Pre-existing Asthma
Patients with asthma may have aspirin-sensitive asthma. The use of aspirin in patients with aspirin-sensitive asthma has been associated with severe bronchospasm, which can be fatal. Since cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, oxycodone hydrochloride and ibuprofen tablets should not be administered to
patients with this form of aspirin sensitivity and should be used with caution in patients with pre-existing asthma.
Aseptic Meningitis
Aseptic meningitis with fever and coma has been observed on rare occasions in patients on ibuprofen as found in oxycodone hydrochloride and ibuprofen tablets. Although it is probably more likely to occur in patients with systemic lupus erythematosus and related connective tissue diseases, it has been reported in patients who do not have an underlying chronic disease. If signs or symptoms of meningitis develop in a patient on oxycodone hydrochloride and ibuprofen tablets, the possibility of its being related to ibuprofen should be considered.
Information for Patients
-
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.
-
Oxycodone hydrochloride and ibuprofen tablets, similar to other opioidcontaining analgesics, may impair mental and/or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery; patients should be cautioned accordingly.
-
The combination of this product with alcohol and other CNS depressants may produce an additive CNS depression and should be avoided.
-
Oxycodone hydrochloride and ibuprofen tablets can be abused in a manner similar to other opioid agonists, legal or illicit. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
-
Oxycodone hydrochloride and ibuprofen tablets, like other NSAIDs, may cause serious CV side effects, such as MI or stroke, which may result in hospitalization and even death. Although serious CV events can occur without warning symptoms, patients should be alert for the signs and symptoms of chest pain, shortness of breath, weakness, slurring of speech, and should ask for medical advice when observing any indicative sign or symptoms. Patients should be apprised of the importance of this follow-up (see WARNINGS; Cardiovascular Effects).
-
Oxycodone hydrochloride and ibuprofen tablets, like other NSAIDs, can cause GI discomfort and, rarely, serious GI side effects, such as ulcers and bleeding, which may result in hospitalization and even death. Although serious GI tract ulcerations and bleeding can occur without warning symptoms, patients should be alert for the signs and symptoms of ulcerations and bleeding, and should ask for medical advice when observing any indicative sign or symptoms including epigastric pain, dyspepsia, melena, and hematemesis. Patients should be apprised of the importance of this follow-up (see WARNINGS; Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation ).
-
Oxycodone hydrochloride and ibuprofen tablets, like other NSAIDs, can cause serious skin side effects such as exfoliative dermatitis, SJS, and TEN, which may result in hospitalizations and even death. Although serious skin reactions may occur without warning, patients should be alert for the signs and symptoms of skin rash and blisters, fever or other signs of hypersensitivity, and should ask medical advice when observing any indicative signs or symptoms. Patients should be advised to stop the drug immediately if they develop any type of rash and contact their physician as soon as possible.
-
Patients should promptly report signs or symptoms of unexplained weight gain or edema to their physicians.
-
Patients should be informed of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, pruritius, jaundice, right upper quadrant tenderness, and “flu-like” symptoms). If these occur, patients should be instructed to seek immediate medical therapy.
-
Patients should be informed of the signs and symptoms of an anaphylactoid reaction (e.g. difficulty breathing, swelling of the face or throat). If these occur, patients should be instructed to seek immediate emergency help (see WARNINGS ).
-
In late pregnancy, as with other NSAIDs, oxycodone hydrochloride and ibuprofen tablets should be avoided because it may cause premature closure of the ductus arteriosus.
Laboratory Tests
Because serious GI tract ulcerations and bleeding can occur without warning symptoms, physicians should monitor for signs or symptoms of GI bleeding. Patients on long-term treatment with NSAIDs should have their CBC and a chemistry profile checked periodically. If clinical signs and symptoms consistent with liver or renal disease develop, systemic manifestations occur (e.g. eosinophilia, rash, etc.) or if abnormal liver tests persist or worsen, oxycodone hydrochloride and ibuprofen tablets should be discontinued.
Drug Interactions
Oxycodone is metabolized in part to oxymorphone via the cytochrome P450 isoenzyme CYP2D6. While this pathway may be blocked by a variety of drugs (e.g., certain cardiovascular drugs and antidepressants), such blockade has not yet been shown to be of clinical significance with this agent. However, clinicians should be aware of this possible interaction.
Anticholinergics: The concurrent use of anticholinergics with oxycodone preparations may produce paralytic ileus.
CNS Depressants: Patients receiving narcotic analgesics, general anesthetics, phenothiazines, other tranquilizers, sedative-hypnotics or other CNS depressants (including alcohol) concomitantly with oxycodone may exhibit an additive CNS depression. Interactive effects resulting in respiratory depression, hypotension, profound sedation, or coma may result if these drugs are taken in combination with the usual dosage of oxycodone. When such combined therapy is contemplated, the dose of one or both agents should be reduced.
Mixed Agonist/Antagonist Opioid Analgesics: Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol and buprenorphine) should be administered with caution to patients who have received or are receiving a course of therapy with a pure opioid agonist analgesic such as oxycodone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxycodone and/or may precipitate withdrawal symptoms in these patients.
Monoamine Oxidase Inhibitors (MAOIs): MAOIs have been reported to intensify the effects of at least one opioid drug causing anxiety, confusion and significant depression of respiration or coma. The use of oxycodone is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
Neuromuscular Blocking Agents: Oxycodone, as well as other opioid analgesics, may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
ACE-Inhibitors: Reports suggest that NSAIDs may diminish the antihypertensive effect of ACE-inhibitors. This interaction should be given consideration in patients taking oxycodone hydrochloride and ibuprofen tablets concomitantly with ACE-inhibitors.
Aspirin: When oxycodone hydrochloride and ibuprofen tablets are administered with aspirin, its protein binding is reduced, although the clearance of free oxycodone hydrochloride and ibuprofen tablets are not altered. The clinical significance of this interaction is not known; however as with other products containing NSAIDs, concomitant administration of oxycodone hydrochloride and ibuprofen tablets and aspirin is not generally recommended because of the potential of increased adverse effects.
Diuretics: Ibuprofen has been shown to reduce the natriuretic effect of furosemide and thiazides in some patients. This response has been attributed to inhibition of renal prostaglandin synthesis. During concomitant therapy with oxycodone hydrochloride and ibuprofen tablets, the patient should be observed closely for signs of renal failure (see WARNINGS; Renal Effects ), as well as diuretic efficacy.
Lithium: Ibuprofen has been shown to produce an elevation of plasma lithium levels and a reduction in renal lithium clearance. The mean minimum lithium concentration increased 15% and the renal clearance was decreased by approximately 20%. These effects have been attributed to inhibition of renal prostaglandin synthesis by the NSAID. Thus, when oxycodone hydrochloride and ibuprofen tablets and lithium are administered concurrently, subjects should be observed carefully for signs of lithium toxicity.
Methotrexate: Ibuprofen, as well as other NSAIDs, has been reported to competitively inhibit methotrexate accumulation in rabbit kidney slices. This may indicate that ibuprofen could enhance the toxicity of methotrexate. Caution should be used when oxycodone hydrochloride and ibuprofen tablets are administered concomitantly with methotrexate.
Warfarin: The effects of warfarin and NSAIDs on GI bleeding are synergistic, such that users of both drugs together have a greater risk of serious GI bleeding than users of either drug alone.
Carcinogenesis, Mutagenesis and Impairment of Fertility
Studies to evaluate the potential effects of the combination of oxycodone and ibuprofen on carcinogenicity, mutagenicity or impairment of fertility have not been conducted.
Pregnancy
Pregnancy Category C
Animal studies to assess the potential effects of the combination of oxycodone and ibuprofen on embryo-fetal development were conducted in the rat and rabbit model.
Pregnant rats were treated by oral gavage with combination doses of oxycodone: ibuprofen mg/kg/day (0.25:20, 0.5:40, 1.0:80, or 2.0:160) on days 7-16 of gestation. There was no evidence for developmental toxicity or teratogenicity at any dose, although maternal toxicity was noted at doses of 0.5:40 and above. The highest dose tested in the rat (2.00:160 mg/kg/day) is equivalent to the maximum recommended human daily dose (20:1600 mg/day) on a body surface area (mg/m2) basis. This dose was associated with maternal toxicity (death, clinical signs, decreased BW).
Pregnant rabbits were treated by oral gavage with combination doses of oxycodone/ibuprofen (0.38:30, 0.75:60, 1.50:120 or 3.00:240 mg/kg/day) on gestation days 7-19. Oxycodone/ibuprofen treatment was not teratogenic under the conditions of the assay. Maternal toxicity was noted at doses of 1.5:120 (reduced body weight and food consumption) and 3:240 mg/kg/day (mortality). The NOAEL for maternal toxicity, 0.75:60 mg/kg/day, is 0.75 fold the proposed maximum daily human dose based upon the body surface area. Developmental toxicity, as evidenced by delayed ossification and reduced fetal body weights, was noted at the highest dose, which is approximately 3 times the MRHD on a mg/m2 basis, and is likely due to maternal toxicity. The fetal no adverse effect level (NOAEL) of 1.50:120 mg/kg/day is approximately 1.5 times the MRHD on a mg/m2 basis.
There are no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. Oxycodone hydrochloride and ibuprofen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Because of the known effects of NSAIDs on the fetal cardiovascular system (closure of the ductus arteriosus), use in pregnancy, particularly late pregnancy should be avoided.
Babies born to mothers who have been taking opioids regularly prior to delivery will be physical dependent. The withdrawal signs include irritability and excessive crying, tremors, hyperactive reflexes, increased respiratory rate, increased stools, sneezing, yawning, vomiting, and fever. The intensity of the syndrome does not always correlate with the duration of maternal opioid use or dose. There is no consensus on the best method of managing withdrawal.
Labor and Delivery
Oxycodone hydrochloride and ibuprofen tablets should not be used during the third trimester of pregnancy due to the potential for ibuprofen to inhibit prostaglandin synthetase which may prolong pregnancy and inhibit labor. Oxycodone is not recommended for use in women during and immediately prior to labor and delivery because oral opioids may cause respiratory depression in the newborn.
In rat studies with NSAIDs, as with other drugs known to inhibit prostaglandin synthesis, an increased incidence of dystocia, delayed parturition, and decreased pup survival occurred. The effects of oxycodone hydrochloride and ibuprofen tablets on labor and delivery in pregnant women are unknown.
Nursing Mothers
It is not known whether oxycodone hydrochloride and ibuprofen tablets are excreted in human milk. Oxycodone is excreted in human milk. Withdrawal symptoms and/or respiratory depression have been observed in neonates whose mothers were taking narcotic analgesics during pregnancy. Although adverse effects in the nursing infant have not been documented, withdrawal can occur in breast-feeding infants when maternal administration of an opioid analgesic is discontinued.
Because many drugs are excreted in human-milk and because of the potential for serious adverse reactions in nursing infants from oxycodone hydrochloride and ibuprofen tablets, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
In the placebo-controlled, clinical studies of pain following dental surgery, 109 patients between the ages of 14 and 17 years were administered a single dose of oxycodone hydrochloride and ibuprofen tablets. No apparent differences were noted in the safety of oxycodone hydrochloride and ibuprofen tablets in patients below and above 17 years of age. Oxycodone hydrochloride and ibuprofen tablets have not been studied in patients under 14 years of age. Safety and effectiveness in pediatric patients below the age of 14 have not been established.
Geriatric Use
Of the total number of subjects in clinical studies of oxycodone hydrochloride and ibuprofen tablets, 89 patients were 65 and over, while 37 patients were 75 and over. No overall differences in safety were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
However, because the elderly may be more sensitive to the renal and gastrointestinal effects of nonsteroidal anti-inflammatory agents as well as possible increased risk of respiratory depression with opioids, extra caution should be used when treating the elderly with oxycodone hydrochloride and ibuprofen tablets.
OXYCODONE HYDROCHLORIDE AND IBUPROFEN ADVERSE REACTIONS
Listed below are the adverse event incidence rates from single dose analgesia trials in which a total of 2437 patients received either oxycodone hydrochloride and ibuprofen tablets, ibuprofen (400 mg), oxycodone hydrochloride (5 mg), or placebo. Adverse event information is also provided from an additional 334 patients who were exposed to oxycodone hydrochloride and ibuprofen tablets in a multiple dose analgesia trial, without placebo or active component comparison arms, given up to four times daily for up to 7 days.
| 5/400 mg
(n=923) |
400 mg
Ibuprofen (n=913) |
5 mg
Oxycodone Hydrochloride (n=286) |
Placebo
(n=315) |
|
| Digestive | ||||
| Nausea | 81 (8.8%) | 44 (4.8%) | 46 (16.1%) | 21 (6.7%) |
| Vomiting | 49 (5.3%) | 16 (1.8%) | 30 (10.5%) | 10 (3.2%) |
| Flatulence | 9 (1.0%) | 7 (0.8%) | 3 (1.0%) | 0 |
| Nervous System | ||||
| Somnolence | 67 (7.3%) | 38 (4.2%) | 12 (4.2%) | 7 (2.2%) |
| Dizziness | 47 (5.1%) | 21 (2.3%) | 17 (5.9%) | 8 (2.5%) |
| Skin and Appendages | ||||
| Sweat | 15 (1.6%) | 7 (0.8%) | 4 (1.4%) | 1 (0.3%) |
Adverse events that were reported by at least 1% of patients taking oxycodone hydrochloride and ibuprofen tablets but were observed at a greater incidence in the placebo treated patients were fever, headache and pruritus. Adverse events that occurred in less than 1% and in at least two oxycodone hydrochloride and ibuprofen tablets treated patients in Single Dose studies not listed above include the following: Body as Whole: abdominal pain, asthenia, chest pain, enlarged abdomen. Cardiovascular System: hypotension, syncope, tachycardia, vasodilation. Digestive System: constipation, dry mouth, dyspepsia, eructation, ileus. Hemic and Lymphatic System: anemia. Metabolic and Nutritional Disorders: edema. Nervous System: euphoria, insomnia, nervousness. Respiratory System: hypoxia, lung disorder, pharyngitis. Urogenital System: urinary retention.
Adverse events that occurred in the Multiple Dose study in at least 2% of patients treated with oxycodone hydrochloride and ibuprofen tablets include the following: Body as Whole: asthenia (3.3%), fever (3.0%), headache (10.2%). Cardiovascular System: vasodilation (3.0%). Digestive System: constipation (4.5%), diarrhea (2.1%), dyspepsia (2.1%), nausea (25.4%), vomiting (4.5%). Nervous System: dizziness (19.2%), somnolence (17.4%).
Adverse events that occurred in less than 2% of and at least two oxycodone hydrochloride and ibuprofen tablets treated patients in the Multiple Dose study not listed previously include the following: Body as Whole: back pain, chills, infection. Cardiovascular System: thrombophlebitis. Hemic and Lymphatic System: ecchymosis. Metabolic and Nutritional Disorders: hypokalemia. Musculoskeletal System: arthritis. Nervous System: abnormal thinking, anxiety, hyperkinesia, hypertonia. Skin and Appendages: rash. Special Senses: amblyopia, taste perversion. Urogenital System: urinary frequency.
DRUG ABUSE AND DEPENDENCE
Oxycodone hydrochloride and ibuprofen tablets contain oxycodone, which is a mu-opioid agonist with an abuse liability similar to other opioid agonists and is a Schedule II controlled substance. Oxycodone hydrochloride and ibuprofen tablets, and other opioids used in analgesia, can be abused and are subject to criminal diversion.
Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease utilizing a multidisciplinary approach, but relapse is common.
“Drug seeking” behavior is very common in addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated “loss” of prescriptions, tampering with prescriptions and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physical dependence usually assumes clinically significant dimensions only after several weeks of continued opioid use, although a mild degree of physical dependence may develop after a few days of opioid therapy. Tolerance, in which increasingly large doses are required in order to produce the same degree of analgesia, is manifested initially by a shortened duration of analgesic effect, and subsequently by decreases in the intensity of analgesia. The rate of development of tolerance varies among patients. Physicians should be aware that abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. Oxycodone hydrochloride and ibuprofen tablets, like other opioids, may be diverted for non-medical use. Record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
Proper assessment of the patient, proper prescribing practices, periodic reevaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
OVERDOSAGE
Following an acute overdosage, toxicity may result from oxycodone and/or ibuprofen.
Signs and Symptoms
Acute overdosage with oxycodone may be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, bradycardia, or hypotension. In severe cases death may occur.
The toxicity of ibuprofen overdose is dependent on the amount of drug ingested and the time elapsed since ingestion, although individual response may vary, necessitating individual evaluation of each case. Although uncommon, serious toxicity and death have been reported in the medical literature with ibuprofen overdosage. The most frequently reported symptoms of ibuprofen overdose include abdominal pain, nausea, vomiting, lethargy, and drowsiness. Other central nervous system symptoms include headache, tinnitus, CNS depression, and seizures. Cardiovascular toxicity, including hypotension, bradycardia, tachycardia, and atrial fibrillation, have also been reported.
Treatment
In the treatment of opioid overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose, as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation. The narcotic antagonist naloxone hydrochloride is a specific antidote against respiratory depression, which may result from overdosage or unusual sensitivity to narcotics including oxycodone. An appropriate dose of naloxone hydrochloride should be administered intravenously with simultaneous efforts at respiratory resuscitation. Since the duration of action of oxycodone may exceed that of the naloxone, the patient should be kept under continuous surveillance and repeated doses of the antagonist should be administered as needed to maintain adequate respiration. Management of hypotension, acidosis and gastrointestinal bleeding may be necessary. In cases of acute overdose, the stomach should be emptied through ipecac-induced emesis or gastric lavage. Orally administered activated charcoal may help in reducing the absorption and reabsorption of ibuprofen. Emesis is most effective if initiated within 30 minutes of ingestion. Induced emesis is not recommended in patients with impaired consciousness or overdoses greater than 400 mg/kg of the ibuprofen component in children because of the risk for convulsions and the potential for aspiration of gastric contents.
OXYCODONE HYDROCHLORIDE AND IBUPROFEN DOSAGE AND ADMINISTRATION
Carefully consider the potential benefits and risks of oxycodone hydrochloride and ibuprofen tablets and other treatment options before deciding to use oxycodone hydrochloride and ibuprofen tablets. Use the lowest effective dose for the shortest duration consistent with individual patient treatment goals (see WARNINGS ).
After observing the response to initial therapy with oxycodone hydrochloride and ibuprofen tablets, the dose and frequency should be adjusted to suit an individual patient’s needs.”
For the management of acute moderate to severe pain, the recommended dose of oxycodone hydrochloride and ibuprofen is one tablet given orally. Dosage should not exceed 4 tablets in a 24-hour period and should not exceed 7 days.
HOW SUPPLIED
Oxycodone Hydrochloride and Ibuprofen Tablets, 5 mg/400 mg are capsule shaped, white to off-white, film-coated tablets with 34|94 on one side and WATSON on the other side. The numbered side of the tablet is bisected.
Bottles of 30, 100 and 500.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
A Schedule CII Narcotic
Manufactured By:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Distributed By:
Watson Pharma, Inc.
Corona, CA 92880 USA
Revised: July 2008
0708B
(See the end of this Medication Guide for a list of prescription NSAID medicines.)
| What is the most important information I should know about medicines called Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)? | ||
| NSAID medicines may increase the chance of a heart attack or stroke that can lead to death. | ||
| This chance increases: | ||
| • with longer use of NSAID medicines | ||
| • in people who have heart disease | ||
| NSAID medicines should never be used right before or after a heart surgery called a “coronary artery bypass graft (CABG).” | ||
| NSAID medicines can cause ulcers and bleeding in the stomach and intestines at any time during treatment. Ulcers and bleeding: | ||
| • can happen without warning symptoms | ||
| • may cause death | ||
| The chance of a person getting an ulcer or bleeding increases with: | ||
| • taking medicines called “corticosteroids” and “anticoagulants” | • smoking | |
| • longer use | • drinking alcohol | |
| • older age | ||
| • having poor health | ||
| NSAID medicines should only be used: | ||
| • exactly as prescribed | ||
| • at the lowest dose possible for your treatment | ||
| • for the shortest time needed | ||
What are Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
NSAID medicines are used to treat pain and redness, swelling, and heat (inflammation) from medical conditions such as:
-
different types of arthritis
-
menstrual cramps and other types of short-term pain
Who should not take a Non-Steroidal Anti-Inflammatory Drug (NSAID)?
Do not take an NSAID medicine:
-
if you had an asthma attack, hives, or other allergic reaction with aspirin or any other NSAID medicine
-
for pain right before or after heart bypass surgery
Tell your healthcare provider:
-
about all your medical conditions.
-
about all of the medicines you take. NSAIDs and some other medicines can interact with each other and cause serious side effects. Keep a list of your medicines to show to your healthcare provider and pharmacist.
-
if you are pregnant. NSAID medicines should not be used by pregnant women late in their pregnancy.
-
if you are breastfeeding. Talk to your doctor.
What are the possible side effects of Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)?
| Serious side effects include: | Other side effects include: |
| • heart attack | • stomach pain |
| • stroke | • constipation |
| • high blood pressure | • diarrhea |
| • heart failure from body swelling (fluid retention) | • gas |
| • kidney problems including kidney failure | • heartburn |
| • bleeding and ulcers in the stomach and intestine | • nausea |
| • low red blood cells (anemia) | • vomiting |
| • life-threatening skin reactions | • dizziness |
| • life-threatening allergic reactions | |
| • liver problems including liver failure | |
| • asthma attacks in people who have asthma |
Get emergency help right away if you have any of the following symptoms:
-
shortness of breath or trouble breathing
-
chest pain
-
weakness in one part or side of your body
-
slurred speech
-
swelling of the face or throat
Stop your NSAID medicine and call your healthcare provider right away if you have any of the following symptoms:
-
nausea
-
more tired or weaker than usual
-
itching
-
your skin or eyes look yellow
-
stomach pain
-
flu-like symptoms
-
vomit blood
-
there is blood in your bowel movement or it is black and sticky like tar
-
unusual weight gain
-
skin rash or blisters with fever
-
swelling of the arms and legs, hands and feet
These are not all the side effects with NSAID medicines. Talk to your healthcare provider or pharmacist for more information about NSAID medicines. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Other information about Non-Steroidal Anti-Inflammatory Drugs (NSAIDs)
-
Aspirin is an NSAID medicine but it does not increase the chance of a heart attack. Aspirin can cause bleeding in the brain, stomach, and intestines. Aspirin can also cause ulcers in the stomach and intestines.
-
Some of these NSAID medicines are sold in lower doses without a prescription (over-the-counter). Talk to your healthcare provider before using over-the-counter NSAIDs for more than 10 days.
NSAID medicines that need a prescription
| Generic Name | Tradename |
| * Vicoprofen contains the same dose of ibuprofen as over-the-counter (OTC) NSAIDs, and is usually used for less than 10 days to treat pain. The OTC NSAID label warns that long term continuous use may increase the risk of heart attack or stroke. | |
| Celecoxib | Celebrex |
| Diclofenac | Cataflam, Voltaren, Arthrotec (combined with misoprostol) |
| Diflunisal | Dolobid |
| Etodolac | Lodine, Lodine XL |
| Fenoprofen | Nalfon, Nalfon 200 |
| Flurbiprofen | Ansaid |
| Ibuprofen | Motrin, Tab-Profen, Vicoprofen* (combined with hydrocodone), Combunox (combined with oxycodone) |
| Indomethacin | Indocin, Indocin SR, Indo-Lemmon, Indomethagan |
| Ketoprofen | Oruvail |
| Ketorolac | Toradol |
| Mefenamic Acid | Ponstel |
| Meloxicam | Mobic |
| Nabumetone | Relafen |
| Naproxen | Naprosyn, Anaprox, Anaprox DS, EC-Naproxyn, Naprelan, Naprapac (copackaged with lansoprazole) |
| Oxaprozin | Daypro |
| Piroxicam | Feldene |
| Sulindac | Clinoril |
| Tolmetin | Tolectin, Tolectin DS, Tolectin 600 |
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured By:
Watson Laboratories, Inc.
Corona, CA 92880 USA
Distributed By:
Watson Pharma, Inc.
Corona, CA 92880 USA
Revised: July 2008
Principal Display Panel
NDC 0591-3494-01
CII
Oxycodone Hydrochloride and Ibuprofen Tablets
5 mg/400 mg
Watson® 100 Tablets Rx only

oxycodone hydrochloride and ibuprofenoxycodone hydrochloride and ibuprofen TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||