Oxcarbazepine
FULL PRESCRIBING INFORMATION: CONTENTS*
- OXCARBAZEPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- USE IN SPECIFIC POPULATIONS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- OXCARBAZEPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- OXCARBAZEPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
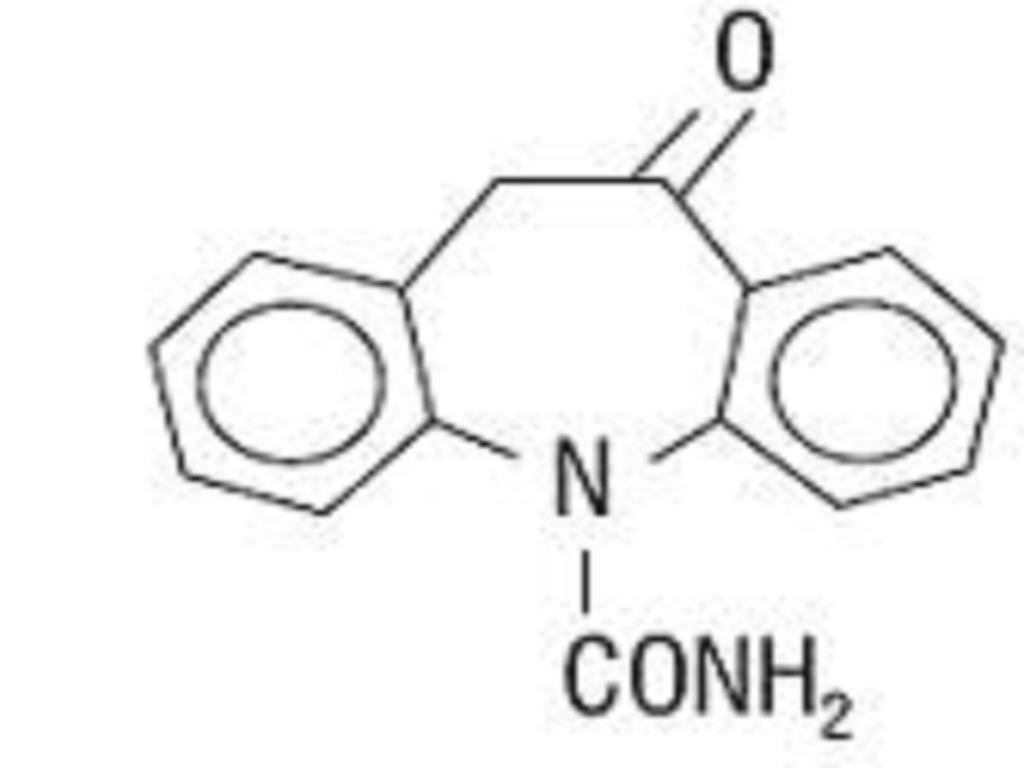
OXCARBAZEPINE DESCRIPTION
CLINICAL PHARMACOLOGY
Mechanism of ActionMetabolism and Excretion subsection
PHARMACODYNAMICS
PHARMACOKINETICS
Distribution
Metabolism and Excretion
USE IN SPECIFIC POPULATIONS
Hepatic ImpairmentRenal Impairment
PRECAUTIONSDOSAGE AND ADMINISTRATION
Pediatric Use
Geriatric Use
Gender
Race
CLINICAL STUDIES
Oxcarbazepine Monotherapy Trials
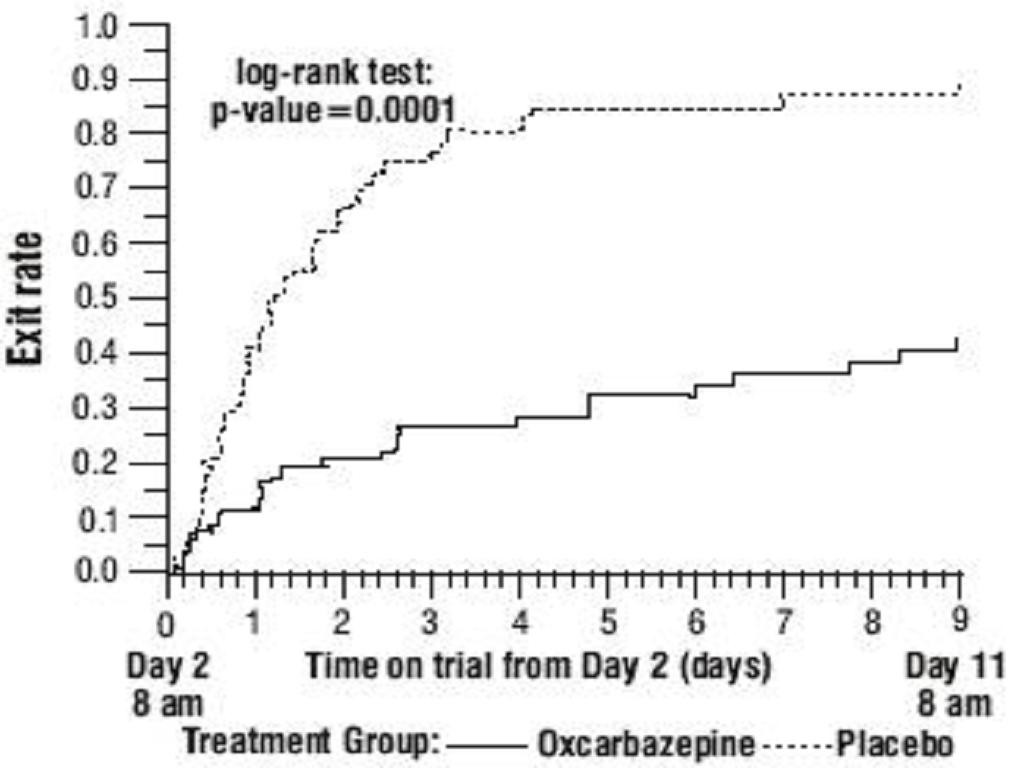
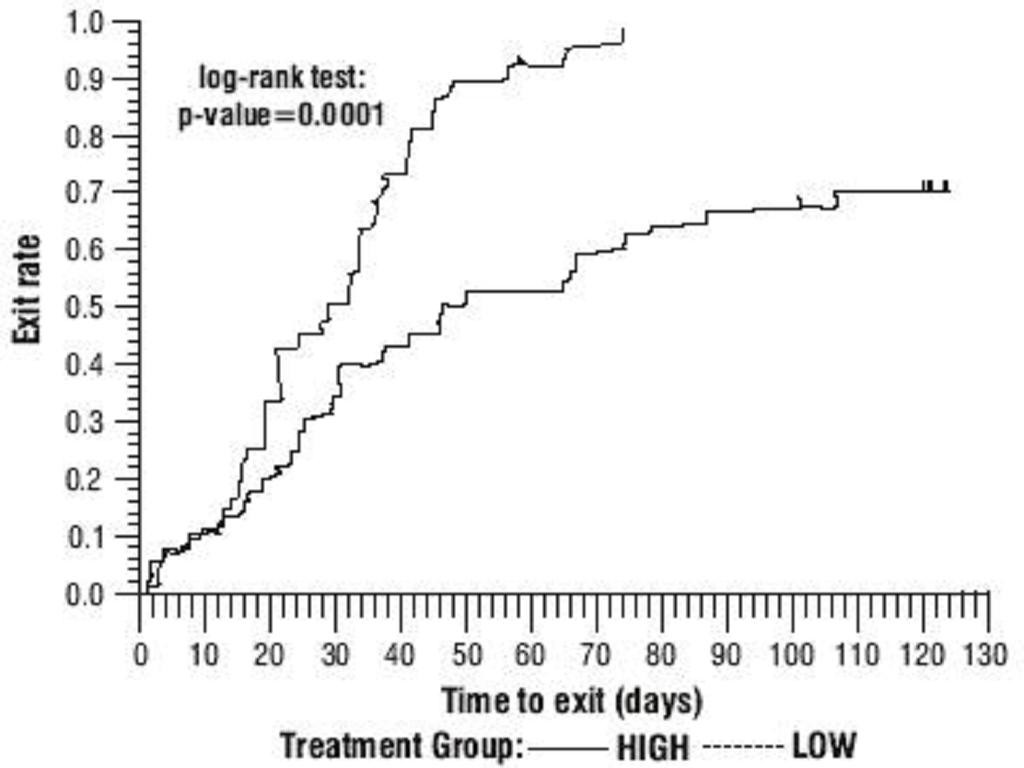
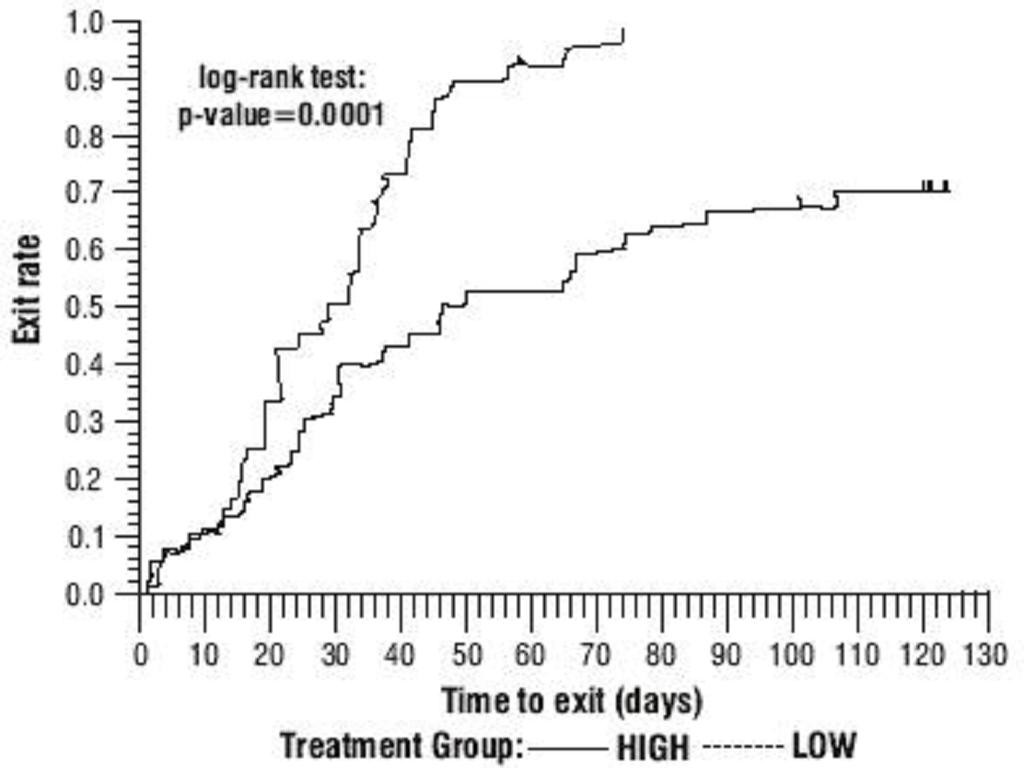
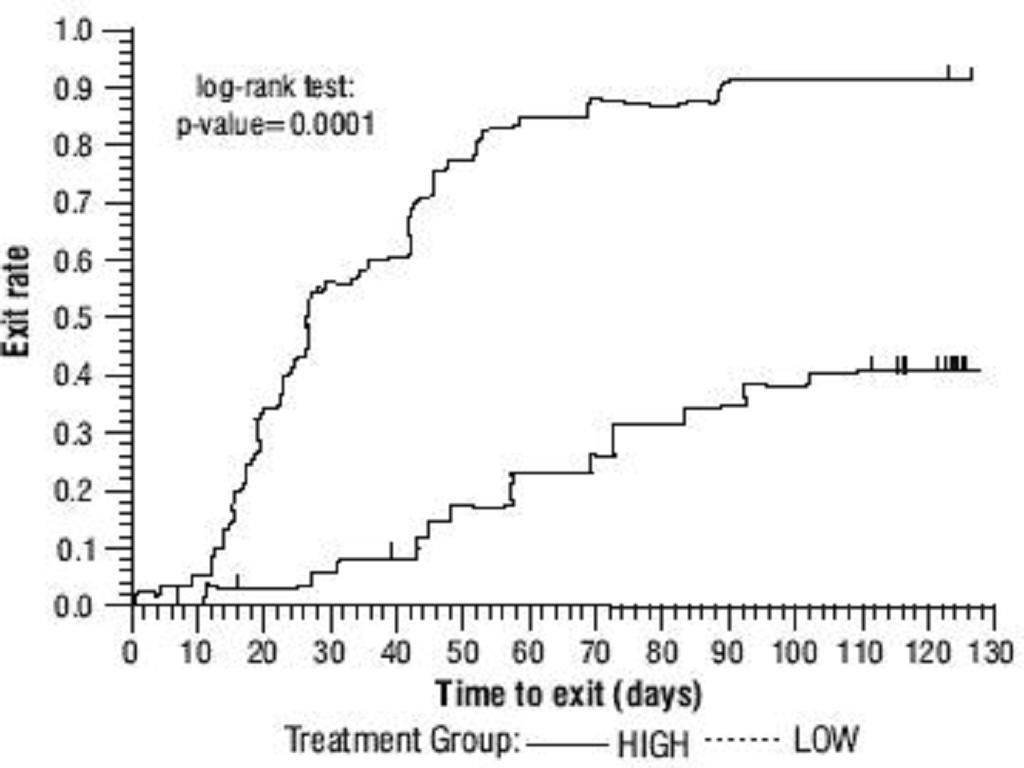
Oxcarbazepine Adjunctive Therapy Trials
Table 1ADVERSE REACTIONSsection), an outcome not seen in the monotherapy studies.
Table 1: Summary of Percentage Change in Partial Seizure Frequency from Baseline for Placebo-Controlled Adjunctive Therapy Trials
TrialTreatment GroupNBaseline Median Seizure Rate*Median % Reduction1 (pediatrics)Oxcarbazepine13612.534.81Placebo12813.19.42 (adults)Oxcarbazepine 2400 mg/day17410.049.91Oxcarbazepine 1200 mg/day1779.840.21Oxcarbazepine 600 mg/day1689.626.41Placebo1738.67.6
Subset analyses of the antiepileptic efficacy of oxcarbazepine with regard to gender in these trials revealed no important differences in response between men and women. Because there were very few patients over the age of 65 in controlled trials, the effect of the drug in the elderly has not been adequately assessed.
The third adjunctive therapy trial enrolled 128 pediatric patients (1 month to <4 years of age) with inadequately-controlled partial seizures on 1 to 2 concomitant AEDs. Patients who experienced at least 2 study-specific seizures (i.e., electrographic partial seizures with a behavioral correlate) during the 72-hour baseline period were randomly assigned to either oxcarbazepine 10 mg/kg/day or were titrated up to 60 mg/kg/day within 26 days. Patients were maintained on their randomized target dose for 9 days and seizures were recorded through continuous video-EEG monitoring during the last 72 hours of the maintenance period. The primary measure of effectiveness in this trial was a between-group comparison of the change in seizure frequency per 24 hours compared to the seizure frequency at baseline. For the entire group of patients enrolled, this comparison was statistically significant in favor of oxcarbazepine 60 mg/kg/day. In this study, there was no evidence that oxcarbazepine was effective in patients below the age of 2 years (N=75).
INDICATIONS & USAGE
OXCARBAZEPINE CONTRAINDICATIONS
WARNINGS
Hyponatremia
Anaphylactic Reactions and Angioedema
WARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
WARNINGS, Anaphylactic Reactions and AngioedemaPRECAUTIONS, Multi-Organ Hypersensitivitysubsection).
Serious Dermatological Reactions
Serious dermatological reactions, including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN), have been reported in both children and adults in association with oxcarbazepine use. The median time of onset for reported cases was 19 days. Such serious skin reactions may be life threatening, and some patients have required hospitalization with very rare reports of fatal outcome. Recurrence of the serious skin reactions following re-challenge with oxcarbazepine has also been reported.
The reporting rate of TEN and SJS associated with oxcarbazepine use, which is generally accepted to be an underestimate due to underreporting, exceeds the background incidence rate estimates by a factor of 3- to 10-fold. Estimates of the background incidence rate for these serious skin reactions in the general population range between 0.5 to 6 cases per million-person years. Therefore, if a patient develops a skin reaction while taking oxcarbazepine tablets, consideration should be given to discontinuing oxcarbazepine use and prescribing another antiepileptic medication.
Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including oxcarbazepine, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% CI:1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence rate of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting drug treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5 to 100 years) in the clinical trials analyzed.Table 2shows absolute and relative risk by indication for all evaluated AEDs.
Table 2 Risk by Indication for Antiepileptic Drugs in the Pooled Analysis
IndicationPlacebo Patients with Events Per 1,000 PatientsDrug Patients with Events Per 1,000 PatientsRelative Risk: Incidence of Events in Drug Patients/Incidence in Placebo PatientsRisk Difference: Additional Drug Patients with Events Per 1,000 PatientsEpilepsy1.03.43.52.4Psychiatric5.78.51.52.9Other1.01.81.90.9Total2.44.31.81.9The relative risk for suicidal thoughts or behavior was higher in clinical trials for epilepsy than in clinical trials for psychiatric or other conditions, but the absolute risk differences were similar for the epilepsy and psychiatric indications.
Anyone considering prescribing oxcarbazepine or any other AED must balance the risk of suicidal thoughts or behavior with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
Withdrawal of AEDs
As with all antiepileptic drugs, oxcarbazepine should be withdrawn gradually to minimize the potential of increased seizure frequency.
PRECAUTIONS
Cognitive/Neuropsychiatric Adverse EventsAdult Patients
Pediatric Patients
Multi-Organ Hypersensitivity
WARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
INFORMATION FOR PATIENTS
WARNINGS, Anaphylactic Reactions and AngioedemaWARNINGS, Patients with a Past History of Hypersensitivity Reaction to Carbamazepine
WARNINGS, Serious Dermatological Reactions
PRECAUTIONS, Multi-organ Hypersensitivity
Drug Interactions
PRECAUTIONS, Pregnancy Category C
LABORATORY TESTS
WARNINGSDRUG INTERACTIONS
Antiepileptic Drugs
Table 3
Hormonal Contraceptives
Drug Interactions
Calcium Antagonists
Other Drug Interactions
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
LABOR & DELIVERY
NURSING MOTHERS
Patients with Renal Impairment
CLINICAL PHARMACOLOGY, Pharmacokinetics
PEDIATRIC USE
ADVERSE REACTIONSGERIATRIC USE
OXCARBAZEPINE ADVERSE REACTIONS
Most Common Adverse Events in All Clinical StudiesTable 4Table 5
Table 6
Table 7
Other Events Observed in Association with the Administration of Oxcarbazepine
Post-Marketing and Other Experience
PRECAUTIONS, Multi-Organ Hypersensitivity
WARNINGS, Anaphylactic Reactions and Angioedema
WARNINGS, Serious Dermatological Reactions
DRUG ABUSE AND DEPENDENCE
AbuseDependence
OVERDOSAGE
Human Overdose ExperienceTreatment and Management
DOSAGE & ADMINISTRATION
CLINICAL PHARMACOLOGY, Pharmacokinetics
Adults
Adjunctive Therapy
PRECAUTIONS, Drug Interactions
Conversion to Monotherapy
Initiation of Monotherapy
Patients not currently being treated with AEDs may have monotherapy initiated with oxcarbazepine. In these patients, oxcarbazepine should be initiated at a dose of 600 mg/day (given in a BID regimen); the dose should be increased by 300 mg/day every third day to a dose of 1200 mg/day. Controlled trials in these patients examined the effectiveness of a 1200mg/day dose; a dose of 2400 mg/day has been shown to be effective in patients converted from other AEDs to oxcarbazepine monotherapy (see above).
Pediatric Patients
Adjunctive Therapy (Aged 2 to 16 Years)
CLINICAL PHARMACOLOGY
Conversion to Monotherapy (Aged 4 to 16 Years)
Initiation of Monotherapy (Aged 4 to 16 Years)
Patients with Hepatic Impairment
CLINICAL PHARMACOLOGYPharmacokineticsSpecial Populations
Patients with Renal Impairment
CLINICAL PHARMACOLOGYPharmacokineticsSpecial Populations
HOW SUPPLIED
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

OxcarbazepineOxcarbazepine TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!