OPANA
HIGHLIGHTS OF PRESCRIBING INFORMATION INDICATIONS AND USAGEOPANA is an opioid agonist indicated for the relief of moderate to severe acute pain where the use of an opioid is appropriate. (1)DOSAGE AND ADMINISTRATION Titrate the dose based on individual response. (2.1) OPANA should be taken on an empty stomach, at least one hour prior to or two hours after eating. (2.2) Opioid-Naïve Patients: 10 to 20 mg orally every four to six hours. Initiation of therapy with doses higher than 20 mg is not recommended. If necessary, may be initiated with 5 mg (e.g., for renal or hepatic impairment, geriatric patients). (2) Conversion to OPANA: Follow recommendations for conversion from other opioids or parenteral oxymorphone. (2.2) Cessation of Therapy: Taper gradually. (2.4) DOSAGE FORMS AND STRENGTHSTablets: 5 mg and 10 mg. (3)CONTRAINDICATIONS Known hypersensitivity to oxymorphone, any other ingredients in OPANA, or morphine analogs (4) Respiratory depression in the absence of resuscitative equipment (4) Acute or severe bronchial asthma or hypercarbia (4) Paralytic ileus (4) Moderate or severe hepatic impairment (4) WARNINGS AND PRECAUTIONS Respiratory depression: Increased risk in elderly, debilitated patients, and those suffering from conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve. (5.1) Misuse, abuse, and diversion: OPANA is an opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine. (5.2) CNS effects: Additive CNS-depressive effects when used in conjunction with alcohol, other opioids, or illicit drugs. (5.3) Head injury: Effects may be markedly exaggerated. Administer with extreme caution. (5.4) Hypotensive effect: Increased risk with compromised ability to maintain blood pressure. Administer with caution to patients in circulatory shock. (5.5) Mild hepatic impairment: Use with caution and at lower doses due to higher plasma concentrations than in patients with normal hepatic function (5.6) Prolonged gastric obstruction: May occur in patients with gastrointestinal obstruction, especially paralytic ileus. (5.8) Sphincter of Oddi: Administer with caution in patients with biliary tract disease. (5.9) Impaired mental/physical abilities: Caution must be used with potentially hazardous activities. (5.10) Side EffectsAdverse reactions (≥2% of patients): Nausea, pyrexia, somnolence, vomiting, pruritus, headache, dizziness, constipation, and confusion. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact Endo Pharmaceuticals Inc. at 1-800-462-3636 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch . DRUG INTERACTIONS CNS depressants: Increased risk of respiratory depression, hypotension, profound sedation, coma or death. When combined therapy with CNS depressant is contemplated, the dose of one or both agents should be reduced. (7.1) Mixed agonist/antagonist opioids (i.e., pentazocine, nalbuphine, and butorphanol): May reduce analgesic effect and/or precipitate withdrawal symptoms. (7.2) Cimetidine: Combination use may precipitate confusion, disorientation, respiratory depression, apnea, seizures. (7.3) Anticholinergics: May result in urinary retention and/or severe constipation, which may lead to paralytic ileus. (7.4) Monoamine oxidase inhibitors (MAOIs): Potentiate the action of opioids. OPANA should not be used in patients taking MAOIs or within 14 days of stopping such treatment. (7.5) USE IN SPECIFIC POPULATIONS Pregnancy: Based on animal data, may cause fetal harm. (8.1) Geriatric patients: OPANA should be used with caution in elderly patients. (8.5)
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
OPANA is indicated for the relief of moderate to severe acute pain where the use of an opioid is appropriate.
Selection of patients for treatment with OPANA should be governed by the same principles that apply to the use of similar opioid analgesics [see Indications and Usage (1)]. Physicians should individualize treatment in every case [see Dosage and Administration (2.1)], using non-opioid analgesics, opioids on an as needed basis, combination products, and chronic opioid therapy in a progressive plan of pain management such as outlined by the World Health Organization, the Agency for Healthcare Research and Quality, and the American Pain Society.
OPANA should be administered on an empty stomach, at least one hour prior to or two hours after eating [see Clinical Pharmacology (12.3)]
As with any opioid drug product, it is necessary to adjust the dosing regimen for each patient individually, taking into account the patient's prior analgesic treatment experience. In the selection of the initial dose of OPANA, attention should be given to the following:
- The total daily dose, potency and specific characteristics of the opioid the patient has been taking previously;
- The relative potency estimate used to calculate the equivalent oxymorphone dose needed;
- The patient’s degree of opioid tolerance;
- The age, general condition, and medical status of the patient;
- Concurrent non-opioid analgesics and other medications;
- The type and severity of the patient's pain;
- The balance between pain control and adverse experiences;
- Risk factors for abuse or addiction, including a prior history of abuse or addiction.
Once therapy is initiated, frequently assess pain relief and other opioid effects. Titrate dose to adequate pain relief (generally mild or no pain). Patients who experience breakthrough pain may require dosage adjustment.
If signs of excessive opioid-related adverse experiences are observed, the next dose may be reduced. Adjust dosing to obtain an appropriate balance between pain relief and opioid-related adverse experiences. If significant adverse events occur before the therapeutic goal of mild or no pain is achieved, the events should be treated aggressively. Once adverse events are adequately managed, continue upward titration to an acceptable level of pain control.
During periods of changing analgesic requirements, including initial titration, frequent contact is recommended between physician, other members of the healthcare team, the patient, and the caregiver/family. Advise patients and family members of the potential common adverse reactions associated with changing opioid doses.
The dosing recommendations below, therefore, can only be considered as suggested approaches to what is actually a series of clinical decisions over time in the management of the pain of each individual patient.
Titrate dose to adequate pain relief (generally mild or no pain).
Opioid-Naïve Patients
Patients who have not been receiving opioid analgesics should be started on OPANA in a dosing range of 10 to 20 mg every four to six hours depending on the initial pain intensity. If deemed necessary to initiate therapy at a lower dose (e.g., for renal or hepatic impairment or for geriatric patients), patients may be started with OPANA 5 mg. The dose should be titrated based upon the individual patient’s response to their initial dose of OPANA. This dose can then be adjusted to an acceptable level of analgesia taking into account the pain intensity and adverse reactions experienced by the patient.
Initiation of therapy with doses higher than 20 mg is not recommended because of potential serious adverse reactions [see Clinical Studies (14.1)].
Conversion from Parenteral Oxymorphone to OPANA
Given OPANA’s absolute oral bioavailability of approximately 10%, patients receiving parenteral oxymorphone may be converted to OPANA by administering 10 times the patient’s total daily parenteral oxymorphone dose as OPANA, in four or six equally divided doses (e.g., [IV dose x 10] divided by 4 or 6). For example, approximately 10 mg of OPANA four times daily may be required to provide pain relief equivalent to a total daily IM dose of 4 mg oxymorphone. Due to patient variability with regard to opioid analgesic response, upon conversion patients should be closely monitored to ensure adequate analgesia and to minimize side effects.
Conversion from Other Oral Opioids to OPANA
For conversion from other opioids to OPANA, physicians and other healthcare professionals are advised to refer to published relative potency information, keeping in mind that conversion ratios are only approximate. In general, it is safest to start OPANA therapy by administering half of the calculated total daily dose of OPANA in 4 to 6 equally divided doses, every 4-6 hours. The initial dose of OPANA can be gradually adjusted until adequate pain relief and acceptable side effects have been achieved.
During therapy, continual re-evaluation of the patient receiving OPANA is important, with special attention to the maintenance of pain control and the relative incidence of side effects associated with therapy. If the level of pain increases, effort should be made to identify the source of increased pain, while adjusting the dose [see Dosage and Administration (2.1)].
When the patient no longer requires therapy with OPANA, doses should be tapered gradually to prevent signs and symptoms of withdrawal in the physically dependent patient [see Drug Abuse and Dependence (9.3)].
OPANA is contraindicated in patients with moderate or severe hepatic impairment. Use OPANA with caution in patients with mild hepatic impairment, starting with the lowest dose (e.g., 5 mg) and titrating slowly while carefully monitoring side effects [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
2.6 Patients with Renal Impairment
There are 57% and 65% increases in oxymorphone bioavailability in patients with moderate and severe renal impairment, respectively; treated with extended-release oxymorphone tablets [see Clinical Pharmacology (12.3)]. Accordingly, OPANA should be administered cautiously and in reduced dosages to patients with creatinine clearance rates less than 50 mL/min.
OPANA, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system (CNS) depressants including sedatives or hypnotics, general anesthetics, phenothiazines, tranquilizers, and alcohol, because respiratory depression, hypotension and profound sedation, coma or death may result [see Warnings and Precautions (5.3) and Drug Interactions (7.1)]. When combined therapy with any of the above medications is considered, the dose of one or both agents should be reduced.
Although no specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, OPANA is not recommended for use in patients who have received MAO inhibitors within 14 days [see Drug Interactions (7.5)].
Exercise caution in the selection of the starting dose of OPANA for an elderly patient by starting at the low end of the dosing range (e.g., 5 mg) [see Use in Specific Populations (8.5)].
The 5 mg dosage form is a blue, round, convex tablet debossed with E612 over 5 on one side and plain on the other.
The 10 mg dosage form is a red, round, convex tablet debossed with E613 over 10 on one side and plain on the other.
- OPANA is contraindicated in patients with a known hypersensitivity to oxymorphone or to any of the other ingredients in OPANA, or with known hypersensitivity to morphine analogs such as codeine.
- OPANA is contraindicated in patients with respiratory depression, except in monitored settings and in the presence of resuscitative equipment.
- OPANA is contraindicated in patients with acute or severe bronchial asthma or hypercarbia.
- OPANA is contraindicated in any patient who has or is suspected of having paralytic ileus [see Warning and Precautions (5.8)].
- OPANA is contraindicated in patients with moderate or severe hepatic impairment [see Warnings and Precautions (5.6)].
Respiratory depression is the chief hazard of OPANA. Respiratory depression may occur more frequently in elderly or debilitated patients as well as in those suffering from conditions accompanied by hypoxia or hypercapnia, when even moderate therapeutic doses may dangerously decrease pulmonary ventilation.
Administer OPANA with extreme caution to patients with conditions accompanied by hypoxia, hypercapnia, or decreased respiratory reserve such as: asthma, chronic obstructive pulmonary disease or cor pulmonale, severe obesity, sleep apnea syndrome, myxedema, kyphoscoliosis, CNS depression, or coma. In these patients, even usual therapeutic doses of oxymorphone may decrease respiratory drive while simultaneously increasing airway resistance to the point of apnea. Consider alternative non-opioid analgesics and use OPANA only under careful medical supervision at the lowest effective dose in such patients.
OPANA contains oxymorphone, a mu opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine. Opioid agonists are sought by drug abusers and people with addiction disorders and are subject to criminal diversion.
Oxymorphone can be abused in a manner similar to other opioid agonists, legal or illicit. This issue should be considered when prescribing or dispensing oxymorphone in situations where the physician or pharmacist is concerned about an increased risk of misuse, abuse, or diversion.
OPANA tablets may be abused by crushing, chewing, snorting, or injecting the product. These practices pose a significant risk to the abuser that could result in overdose and death [see Drug Abuse and Dependence (9)].
OPANA may be targeted for theft and diversion. Healthcare professionals should contact their State Medical Board, State Board of Pharmacy, or State Control Board for information on how to detect or prevent diversion of this product, and security requirements for storing and handling of OPANA.
Healthcare professionals should advise patients to store OPANA in a secure place, preferably locked and out of the reach of children and other non-caregivers.
Concerns about abuse, misuse, diversion and addiction should not prevent the proper management of pain.
The concomitant use of other CNS depressants including other opioids, general anesthetics, phenothiazines, other tranquilizers, sedatives, hypnotics, and alcohol with oxymorphone may produce increased depressant effects including hypoventilation, hypotension, profound sedation, coma and death [see Drug Interactions (7.1)].
In the presence of head injury, intracranial lesions or a preexisting increase in intracranial pressure, the respiratory depressant effects of opioid analgesics and their potential to elevate cerebrospinal fluid pressure (resulting from vasodilation following CO2 retention) may be markedly exaggerated. Furthermore, opioid analgesics can produce effects on papillary response and consciousness, which may obscure neurologic signs of further increases in intracranial pressure in patients with head injuries.
Administer OPANA with extreme caution in patients who may be particularly susceptible to the intracranial effects of CO2 retention, such as those with evidence of increased intracranial pressure or impaired consciousness. Opioids may obscure the clinical course of a patient with a head injury and should be used only if clinically warranted.
OPANA, like all opioid analgesics, may cause severe hypotension in a patient whose ability to maintain blood pressure has been compromised by a depleted blood volume, or after concurrent administration with drugs such as phenothiazines or other agents that compromise vasomotor tone. Administer OPANA with caution to patients in circulatory shock, since vasodilation produced by the drug may further reduce cardiac output and blood pressure.
A study of extended-release oxymorphone tablets in patients with hepatic disease indicated greater plasma concentrations than in those with normal hepatic function [see Clinical Pharmacology (12.3)]. Use OPANA with caution in patients with mild impairment, starting with the lowest dose and titrating slowly while carefully monitoring for side effects [see Dosage and Administration (2.2, 2.5)]. OPANA is contraindicated in patients with moderate or severe hepatic impairment.
Use OPANA with caution in the following conditions: adrenocortical insufficiency (e.g., Addison’s disease), prostatic hypertrophy or urethral stricture, severe impairment of pulmonary or renal function, and toxic psychosis.
Opioids may aggravate convulsions in patients with convulsive disorders, and may induce or aggravate seizures in some clinical settings.
Opioids diminish propulsive peristaltic waves in the gastrointestinal tract. Monitor for decreased bowel motility in post-operative patients receiving opioids. The administration of OPANA may obscure the diagnosis or clinical course in patients with acute abdominal conditions. OPANA is contraindicated in patients with paralytic ileus.
OPANA, like other opioids, may cause spasm of the sphincter of Oddi and should be used with caution in patients with biliary tract disease, including acute pancreatitis.
Opioid analgesics impair the mental and physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery.
The following serious adverse reactions are discussed elsewhere in the labeling:
- Respiratory depression [see Warnings and Precautions (5.1)]
- Misuse and abuse [see Warnings and Precautions (5.2) and Drug Abuse and Dependence (9)]
- CNS depressant effects [see Warnings and Precautions (5.3)]
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
A total of 591 patients were treated with OPANA in controlled clinical trials. The clinical trials consisted of patients with acute post-operative pain (n=557) and cancer pain (n=34) trials.
The following table lists adverse reactions that were reported in at least 2% of patients receiving OPANA in placebo-controlled trials (acute post-operative pain (N=557)).
Table 1: Adverse Reactions Reported in Placebo-Controlled Trials
| MedDRA Preferred Term | OPANA (N=557) | Placebo (N=270) |
| Nausea | 19% | 12% |
| Pyrexia | 14% | 8% |
| Somnolence | 9% | 2% |
| Vomiting | 9% | 7% |
| Pruritus | 8% | 4% |
| Headache | 7% | 4% |
| Dizziness (ExcudingVertigo) | 7% | 2% |
| Constipation | 4% | 1% |
| Confusion | 3% | <1% |
The common (≥1% - >10%) adverse drug reactions reported at least once by patients treated with OPANA in the clinical trials organized by MedDRA’s (Medical Dictionary for Regulatory Activities) System Organ Class were and not represented in Table 1:
Cardiac disorders: tachycardia
Gastrointestinal disorders: dry mouth, abdominal distention, and flatulence
General disorders and administration site conditions: sweating increased
Nervous system disorders: anxiety and sedation
Respiratory, thoracic and mediastinal disorders: hypoxia
Vascular disorders: hypotension
Other less common adverse reactions known with opioid treatment that were seen <1% in the OPANA trials includes the following:
Abdominal pain, ileus, diarrhea, agitation, disorientation, restlessness, feeling jittery, hypersensitivity, allergic reactions, bradycardia, central nervous system depression, depressed level of consciousness, lethargy, mental impairment, mental status changes, fatigue, depression, clamminess, flushing, hot flashes, dehydration, dermatitis, dyspepsia, dysphoria, edema, euphoric mood, hallucination, hypertension, insomnia, miosis, nervousness, palpitation, postural hypotension, syncope, dyspnea, respiratory depression, respiratory distress, respiratory rate decreased, oxygen saturation decreased, difficult micturition, urinary retention, urticaria, vision blurred, visual disturbances, weakness, appetite decreased, and weight decreased.
The following adverse reactions have been identified during post approval use of OPANA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Nervous system disorder: amnesia, convulsion, memory impairment
The concomitant use of other CNS depressants including sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and alcohol may produce additive CNS depressant effects. OPANA, like all opioid analgesics, should be started at 1/3 to 1/2 of the usual dose in patients who are concurrently receiving other central nervous system depressants because respiratory depression, hypotension, and profound sedation, coma and death may result and titrated slowly as necessary for adequate pain relief.
When combined therapy with any of the above medications is considered, the dose of one or both agents should be reduced [see Dosage and Administration (2.7) and Warnings and Precautions (5.3)].
Agonist/antagonist analgesics (i.e., pentazocine, nalbuphine, butorphanol, and buprenorphine) should be administered with caution to a patient who has received or is receiving a course of therapy with a pure opioid agonist analgesic such as oxymorphone. In this situation, mixed agonist/antagonist analgesics may reduce the analgesic effect of oxymorphone and/or may precipitate withdrawal symptoms in these patients.
CNS side effects have been reported (e.g., confusion, disorientation, respiratory depression, apnea, seizures) following coadministration of cimetidine with opioid analgesics; a causal relationship has not been established.
Anticholinergics or other medications with anticholinergic activity when used concurrently with opioid analgesics may result in increased risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
Opana is not recommended for use in patients who have received MAO inhibitors within 14 days, because severe and unpredictable potentiation by MAO inhibitors has been reported with opioid analgesics. No specific interaction between oxymorphone and monoamine oxidase inhibitors has been observed, but caution in the use of any opioid in patients taking this class of drugs is appropriate.
The safety of using oxymorphone in pregnancy has not been established with regard to possible adverse effects on fetal development. The use of OPANA in pregnancy, in nursing mothers, or in women of child-bearing potential requires that the possible benefits of the drug be weighted against the possible hazards to the mother and the child.
Teratogenic Effects
Pregnancy Category C
There are no adequate and well-controlled studies of oxymorphone in pregnant women. In animal studies, oxymorphone caused decreased fetal and pup weights, an increase in stillbirth, and a decrease in postnatal pup survival at maternal oxymorphone doses equivalent to 0.4 to 4 times the human daily dose of 120 mg (Based on body surface area). OPANA should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
In embryo-fetal developmental toxicity studies, pregnant rats and rabbits received oxymorphone hydrochloride at doses up to about 2 times (rats) and 8 times (rabbits) total human daily dose of 120 mg (based on body surface area). No malformations occurred, but reduced fetal weights occurred at maternal doses of 0.8 (rat) and 4 (rabbit) times the total human daily dose of 120 mg (based on body surface area). There were no adverse developmental effects in rats that received 0.4 times or rabbits that received less than 4 times the total human dose. There were no effects of oxymorphone hydrochloride on intrauterine survival at doses in rats ≤2 times, or in rabbits at ≤8 times the human dose (see Non-teratogenic Effects, below). In a study conducted prior to the establishment of Good Laboratory Practices (GLP) and not according to current recommended methodology, a single subcutaneous injection of oxymorphone hydrochloride on gestation day 8 produced malformations in offspring of hamsters that received a dose equivalent to 10 times the total human daily dose of 120 mg (based on body surface area). This dose also produced 83% maternal lethality.
Non-teratogenic Effects
Oxymorphone hydrochloride administration to female rats during gestation in a pre- and postnatal developmental toxicity study reduced mean litter size (18%) at a dose of 25 mg/kg/day, attributed to an increase in the incidence of stillborn pups. An increase in neonatal death occurred at doses ≥5 mg/kg/day (0.4 times a total human daily dose of 120 mg, based on body surface area). Low pup birth weight, decreased post-natal weight gain, and reduced post-natal survival of pups occurred following treatment of the dams with 25 mg/kg/day (about 2 times a total human daily dose of 120 mg, based on body surface area).
Prolonged use of opioid analgesics during pregnancy may cause fetal-neonatal physical dependence. Neonatal withdrawal may occur. Symptoms usually appear during the first days of life and may include convulsions, irritability, excessive crying, tremors, hyperactive reflexes, fever, vomiting, diarrhea, sneezing, yawning, and increased respiratory rate.
Opioids cross the placenta and may produce respiratory depression in neonates. OPANA is not recommended for use in women during and immediately prior to labor, when use of shorter acting analgesics or other analgesic techniques are more appropriate. Occasionally, opioid analgesics may prolong labor through actions which temporarily reduce the strength, duration, and frequency of uterine contractions. However this effect is not consistent and may be offset by an increased rate of cervical dilatation, which tends to shorten labor. Neonates whose mothers received opioid analgesics during labor should be observed closely for signs of respiratory depression. A specific opioid antagonist, such as naloxone or nalmefene, should be available for reversal of opioid-induced respiratory depression in the neonate.
It is not known whether oxymorphone is excreted in human milk. Because many drugs, including some opioids, are excreted in human milk, caution should be exercised when OPANA is administered to a nursing woman. Infants exposed to OPANA through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breast-fed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Safety and effectiveness of OPANA in pediatric patients below the age of 18 years have not been established.
OPANA should be used with caution in elderly patients [see Clinical Pharmacology (12.3)].
Of the total number of subjects in clinical studies of OPANA, 31% were 65 and over, while 7% were 75 and over. No overall differences in effectiveness were observed between these subjects and younger subjects. There were several adverse events that were more frequently observed in subjects 65 and over compared to younger subjects. These adverse events included dizziness, somnolence, confusion, and nausea. In general, dose selection for elderly patients should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy
In a study of extended-release oxymorphone tablets, patients with mild hepatic impairment were shown to have an increase in bioavailability of 1.6 fold. OPANA should be used with caution in patients with mild impairment. These patients should be started with the lowest dose and titrated slowly while carefully monitoring for side effects. OPANA is contraindicated for patients with moderate and severe hepatic impairment [see Contraindications (4), Warnings and Precautions (5.6), and Dosage and Administration (2.5)].
In a study of extended-release oxymorphone tablets, patients with moderate to severe renal impairment were shown to have an increase in bioavailability ranging from 57-65% [see Clinical Pharmacology (12.3)]. Such patients should be started cautiously with lower doses of OPANA and titrated slowly while monitoring for side effects [see Dosage and Administration (2.6)].
OPANA contains oxymorphone, a mu opioid agonist and a Schedule II controlled substance with an abuse liability similar to morphine and other opioids. Oxymorphone can be abused and is subject to criminal diversion [see Warnings and Precautions (5.2)].
All patients treated with opioids require careful monitoring for signs of abuse and addiction, since use of opioid analgesic products carries the risk of addiction even under appropriate medical use. Addiction is a primary, chronic, neurobiologic disease, with genetic, psychosocial, and environmental factors influencing its development and manifestations. Addiction is characterized by one or more of the following: impaired control over drug use, compulsive use, use for non-medical purposes, and continued use despite harm. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
“Drug-seeking” behavior is very common to addicts and drug abusers. Drug-seeking tactics include emergency calls or visits near the end of office hours, refusal to undergo appropriate examination, testing or referral, repeated claims of loss of prescriptions, tampering with prescriptions, and reluctance to provide prior medical records or contact information for other treating physician(s). “Doctor shopping” (visiting multiple prescribers) to obtain additional prescriptions is common among drug abusers and people suffering from untreated addiction. Preoccupation with achieving adequate pain relief can be appropriate behavior in a patient with poor pain control.
Abuse and addiction are separate and distinct from physical dependence and tolerance. Physicians should be aware that addiction may not be accompanied by concurrent tolerance and symptoms of physical dependence in all addicts. In addition, abuse of opioids can occur in the absence of true addiction and is characterized by misuse for non-medical purposes, often in combination with other psychoactive substances. OPANA, like other opioids, may be diverted for non-medical use. Careful record-keeping of prescribing information, including quantity, frequency, and renewal requests is strongly advised.
OPANA is intended for oral use only. Abuse of OPANA poses a risk of overdose and death. This risk is increased with concurrent abuse of OPANA with alcohol and other substances. Parenteral drug abuse is commonly associated with transmission of infectious diseases such as hepatitis and HIV.
Proper assessment of the patient, proper prescribing practices, periodic re-evaluation of therapy, and proper dispensing and storage are appropriate measures that help to limit abuse of opioid drugs.
Opioid analgesics may cause physical dependence. Physical dependence results in withdrawal symptoms after abrupt discontinuation of a drug or upon administration of an opioid antagonist or mixed opioid agonist/antagonist agent. Withdrawal also may be precipitated through the administration of drugs with opioid antagonist activity, e.g., naloxone, nalmefene, or mixed agonist/antagonist analgesics (pentazocine, butorphanol, buprenorphine, nalbuphine). Physical dependence may not occur to a clinically significant degree until after several days to weeks of continued opioid usage.
Tolerance is the need for increasing doses of opioids to maintain a defined effect such as analgesia (in the absence of disease progression or other external factors). The development of physical dependence and/or tolerance is not unusual during chronic opioid therapy.
OPANA should not be abruptly discontinued [see Dosage and Administration (2.4)]. If OPANA is abruptly discontinued in a physically-dependent patient, an abstinence syndrome may occur. Some or all of the following can characterize this syndrome: restlessness, lacrimation, rhinorrhea, yawning, perspiration, chills, myalgia, and mydriasis. Other symptoms also may develop, including: irritability, anxiety, backache, joint pain, weakness, abdominal cramps, insomnia, nausea, anorexia, vomiting, diarrhea, or increased blood pressure, respiratory rate, or heart rate.
Infants born to mothers physically dependent on opioids will also be physically dependent and may exhibit respiratory difficulties and withdrawal symptoms [see Use in Specific Populations (8.1, 8.2)].
Acute overdosage with OPANA is characterized by respiratory depression (a decrease in respiratory rate and/or tidal volume, Cheyne-Stokes respiration, cyanosis), extreme somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and sometimes bradycardia and hypotension. In some cases, apnea, circulatory collapse, cardiac arrest, and death may occur.
OPANA may cause miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Clinical Pharmacology (12.2)].
In the treatment of OPANA overdosage, primary attention should be given to the re-establishment of a patent airway and institution of assisted or controlled ventilation. Supportive measures (including oxygen and vasopressors) should be employed in the management of circulatory shock and pulmonary edema accompanying overdose as indicated. Cardiac arrest or arrhythmias may require cardiac massage or defibrillation.
The opioid antagonist naloxone hydrochloride is a specific antidote against respiratory depression that may result from overdosage or unusual sensitivity to opioids including OPANA. Nalmefene is an alternative pure opioid antagonist, which may be administered as a specific antidote to respiratory depression resulting from opioid overdose. Since the duration of action of OPANA may exceed that of the antagonist, keep the patient under continued surveillance and administer repeated doses of the antagonist according to the antagonist labeling as needed to maintain adequate respiration.
In patients receiving OPANA, opioid antagonists should not be administered in the absence of clinically significant respiratory or circulatory depression. Administer opioid antagonists cautiously to persons who are known, or suspected to be, physically dependent on any opioid agonist including OPANA. In such cases, an abrupt or complete reversal of opioid effects may precipitate an acute abstinence syndrome. In an individual physically dependent on opioids, administration of the usual dose of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal syndrome produced will depend on the degree of physical dependence and the dose of the antagonist administered. If respiratory depression is associated with muscular rigidity, administration of a neuromuscular blocking agent may be necessary to facilitate assisted or controlled ventilation. Muscular rigidity may also respond to opioid antagonist therapy.
OPANA (oxymorphone hydrochloride) is a semi-synthetic opioid analgesic supplied in 5 mg and 10 mg tablet strengths for oral administration. The tablet strengths describe the amount of oxymorphone hydrochloride per tablet. The tablets contain the following inactive ingredients: lactose monohydrate, magnesium stearate, and pregelatinized starch. In addition, the 5 mg tablets contain FD&C blue No. 2 aluminum lake. The 10 mg tablets contain D&C red No. 30 aluminum lake.
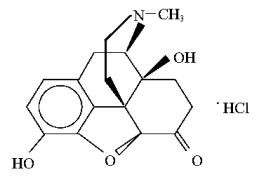
Chemically, oxymorphone hydrochloride is 4, 5α-epoxy-3, 14-dihydroxy-17-methylmorphinan-6-one hydrochloride, a white or slightly off-white, odorless powder, which is sparingly soluble in alcohol and ether, but freely soluble in water. The molecular weight of oxymorphone hydrochloride is 337.80. The pKa1 and pKa2 of oxymorphone at 37°C are 8.17 and 9.54, respectively. The octanol/aqueous partition coefficient at 37°C and pH 7.4 is 0.98.
The structural formula for oxymorphone hydrochloride is as follows:
Oxymorphone, a pure opioid agonist, is relatively selective for the mu receptor, although it can interact with other opioid receptors at higher doses.
The precise mechanism of analgesia, the principal therapeutic action of oxymorphone, is unknown. Specific CNS opiate receptors and endogenous compounds with morphine-like activity have been identified throughout the brain and spinal cord and are likely to play a role in the expression and perception of analgesic effects.
Pharmacological effects of opioid agonists include analgesia, anxiolysis, euphoria, feelings of relaxation, respiratory depression, constipation, miosis, and cough suppression. Like all pure opioid agonist analgesics, with increasing doses there is increasing analgesia, unlike with mixed agonist/antagonists or non-opioid analgesics, where there is a limit to the analgesic effect with increasing doses. With pure opioid agonist analgesics, there is no defined maximum dose; the ceiling to analgesic effectiveness is imposed only by side effects, the more serious of which may include somnolence and respiratory depression.
Concentration-Efficacy Relationships
The minimum effective plasma concentration of oxymorphone for analgesia varies widely among patients, especially among patients who have been previously treated with potent agonist opioids. As a result, individually titrate patients to achieve a balance between therapeutic and adverse effects. The minimum effective analgesic concentration of oxymorphone for any individual patient may increase over time due to an increase in pain, progression of disease, development of a new pain syndrome and/or development of analgesic tolerance.
Concentration-Adverse Experience Relationships
There is a general relationship between increasing opioid plasma concentration and increasing frequency of adverse experiences such as nausea, vomiting, CNS effects, and respiratory depression.
As with all opioids, the dose of OPANA must be individualized [see Dosage and Administration (2.2)]. The effective analgesic dose for some patients will be too high to be tolerated by other patients.
Effects on the Central Nervous System (CNS)
The principal therapeutic action of oxymorphone is analgesia. In common with other opioids, oxymorphone causes respiratory depression, in part by a direct effect on the brainstem respiratory centers. The respiratory depression involves a reduction in the responsiveness of the brain stem respiratory centers to both increases in carbon dioxide tension and electrical stimulation. Opioids depress the cough reflex by direct effect on the cough center in the medulla. Antitussive effects may occur with doses lower than those usually required for analgesia. Oxymorphone causes miosis, even in total darkness. Pinpoint pupils are a sign of opioid overdose but are not pathognomonic (e.g., pontine lesions of hemorrhagic or ischemic origin may produce similar findings). Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations [see Overdosage (10.1)]. Other therapeutic effects of oxymorphone include anxiolysis, euphoria and feelings of relaxation.
In addition to analgesia, the widely diverse effects of oxymorphone include drowsiness, changes in mood, decreased gastrointestinal motility, nausea, vomiting, and alterations of the endocrine and autonomic nervous system.
Effects on the Gastrointestinal Tract and on Other Smooth Muscle
Gastric, biliary and pancreatic secretions are decreased by oxymorphone. Oxymorphone causes a reduction in motility and is associated with an increase in tone in the antrum of the stomach and duodenum. Digestion of food in the small intestine is delayed and propulsive contractions are decreased. Propulsive peristaltic waves in the colon are decreased, while tone is increased to the point of spasm. The end result may be constipation. Oxymorphone can cause a marked increase in biliary tract pressure as a result of spasm of the sphincter of Oddi; and transient elevations in serum amylase. Oxymorphone may also cause spasm of the sphincter of the urinary bladder.
Cardiovascular System Effects
Opioids produce peripheral vasodilation which may result in orthostatic hypotension. Release of histamine can occur and may contribute to opioid-induced hypotension. Manifestations of histamine release may include orthostatic hypotension, pruritus, flushing, red eyes, and sweating. Animal studies have shown that oxymorphone has a lower propensity to cause histamine release than other opioids.
Endocrine System Effects
Opioid agonists have been shown to have a variety of effects on the secretion of hormones. Opioids inhibit the secretion of ACTH, cortisol, and luteinizing hormone (LH) in humans. They also stimulate prolactin, growth hormone (GH) secretion, and pancreatic secretion of insulin and glucagon in humans and other species, rats and dogs. Thyroid stimulating hormone (TSH) has been shown to be both inhibited and stimulated by opioids.
Immune System Effects
Opioids have been shown to have a variety of effects on components of the immune system in in vitro and animal models. The clinical significance of these findings is unknown.
Absorption
The absolute oral bioavailability of oxymorphone is approximately 10%. Studies in healthy volunteers reveal predictable relationships between OPANA dosage and plasma oxymorphone concentrations.
Steady-state levels were achieved after three days of multiple dose administration. Under both single-dose and steady-state conditions, dose proportionality has been established for 5 mg, 10 mg and 20 mg doses of OPANA, for both peak plasma levels (Cmax) and extent of absorption (AUC) (see Table 2).
Table 2
Mean (±SD) OPANA Pharmacokinetic Parameters
| NA = not applicable a Results after 5 days of every 6 hours dosing. |
||||
| Regimen | Dosage | Cmax
(ng/mL) |
AUC (ng•hr/mL) |
T1/2
(hr) |
| Single Dose | 5 mg 10 mg 20 mg |
1.10±0.55 1.93±0.75 4.39±1.72 |
4.48±2.07 9.10±3.40 20.07±5.80 |
7.25±4.40 7.78±3.58 9.43±3.36 |
| Multiple Dose a | 5 mg 10 mg 20 mg |
1.73±0.62 3.51±0.91 7.33±2.93 |
4.63±1.49 10.19±3.34 21.10±7.59 |
NA NA NA |
Food Effect
After oral dosing with 40 mg of OPANA in healthy volunteers under fasting conditions or with a high-fat meal, the Cmax and AUC were increased by approximately 38% in fed subjects relative to fasted subjects. As a result, OPANA should be dosed at least one hour prior to or two hours after eating [see Dosage and Administration (2)].
Ethanol Effect
The effect of co-ingestion of alcohol with OPANA has not been evaluated. However, an in vivo study was performed to evaluate the effect of alcohol (40%, 20%, 4% and 0%) on the bioavailability of a single dose of 40 mg of extended-release oxymorphone tablets in healthy, fasted volunteers. Following concomitant administration of 240 mL of 40% ethanol the Cmax increased on average by 70% and up to 270% in individual subjects. Following the concomitant administration of 240 mL of 20% ethanol, the Cmax increased on average by 31% and up to 260% in individual subjects. In some individuals there was also a decrease in oxymorphone peak plasma concentrations. No effect on the release of oxymorphone from the extended-release tablet was noted in an in vitro alcohol interaction study. The mechanism of the in vivo interaction is unknown. Therefore, avoid co-administration of oxymorphone and ethanol.
Distribution
Formal studies on the distribution of oxymorphone in various tissues have not been conducted. Oxymorphone is not extensively bound to human plasma proteins; binding is in the range of 10% to 12%.
Metabolism
Oxymorphone is highly metabolized, principally in the liver, and undergoes reduction or conjugation with glucuronic acid to form both active and inactive products. The two major metabolites of oxymorphone are oxymorphone-3-glucuronide and 6-OH-oxymorphone. The mean plasma AUC for oxymorphone-3-glucuronide is approximately 90-fold higher than the parent compound. The pharmacologic activity of the glucuronide metabolite has not been evaluated. 6-OH-oxymorphone has been shown in animal studies to have analgesic bioactivity. The mean plasma 6-OH-oxymorphone AUC is approximately 70% of the oxymorphone AUC following single oral doses but is essentially equivalent to the parent compound at steady-state.
Excretion
Because oxymorphone is extensively metabolized, <1% of the administered dose is excreted unchanged in the urine. On average, 33% to 38% of the administered dose is excreted in the urine as oxymorphone-3-glucuronide and 0.25% to 0.62% is excreted as 6-OH-oxymorphone in subjects with normal hepatic and renal function. In animals given radiolabeled oxymorphone, approximately 90% of the administered radioactivity was recovered within 5 days of dosing. The majority of oxymorphone-derived radioactivity was found in the urine and feces.
Pharmacokinetics in Special Populations
Elderly
The plasma levels of oxymorphone administered as an extended-release tablet were about 40% higher in elderly (≥65 years of age) than in younger subjects [see Use in Specific Populations (8.5)].
Gender
The effect of gender on the pharmacokinetics of OPANA has not been studied. In a study with an extended-release formulation of oxymorphone, there was a consistent tendency for female subjects to have slightly higher AUCss and Cmax values than male subjects. However, gender differences were not observed when AUCss and Cmax were adjusted by body weight.
Hepatic Impairment
The liver plays an important role in the pre-systemic clearance of orally administered oxymorphone. Accordingly, the bioavailability of orally administered oxymorphone may be markedly increased in patients with moderate to severe liver disease. The effect of hepatic impairment on the pharmacokinetics of OPANA has not been studied. However, in a study with an extended-release formulation of oxymorphone, the disposition of oxymorphone was compared in 6 patients with mild, 5 patients with moderate, and one patient with severe hepatic impairment, and 12 subjects with normal hepatic function. The bioavailability of oxymorphone was increased by 1.6-fold in patients with mild hepatic impairment and by 3.7-fold in patients with moderate hepatic impairment. In one patient with severe hepatic impairment, the bioavailability was increased by 12.2-fold. The half-life of oxymorphone was not significantly affected by hepatic impairment.
Renal Impairment
The effect of renal impairment on the pharmacokinetics of OPANA has not been studied. However, in a study with an extended-release formulation of oxymorphone, an increase of 26%, 57%, and 65% in oxymorphone bioavailability was observed in mild (creatinine clearance 51-80 mL/min; n=8), moderate (creatinine clearance 30-50 mL/min; n=8), and severe (creatinine clearance <30 mL/min; n=8) patients, respectively, compared to healthy controls.
Drug-Drug Interactions
In vitro studies revealed little to no biotransformation of oxymorphone to 6-OH-oxymorphone by any of the major cytochrome P450 (CYP P450) isoforms at therapeutically relevant oxymorphone plasma concentrations.
No inhibition of any of the major CYP P450 isoforms was observed when oxymorphone was incubated with human liver microsomes at concentrations of ≤50 µM. An inhibition of CYP 3A4 activity occurred at oxymorphone concentrations ≥150 µM. Therefore, it is not expected that oxymorphone, or its metabolites will act as inhibitors of any of the major CYP P450 enzymes in vivo.
Increases in the activity of the CYP 2C9 and CYP 3A4 isoforms occurred when oxymorphone was incubated with human hepatocytes. However, clinical drug interaction studies with OPANA ER showed no induction of CYP450 3A4 or 2C9 enzyme activity, indicating that no dose adjustment for CYP 3A4- or 2C9-mediated drug-drug interactions is required.
Carcinogenesis
Long-term studies have been completed to evaluate the carcinogenic potential of oxymorphone in both Sprague-Dawley rats and CD-1 mice. Oxymorphone was administered to Sprague-Dawley rats (2.5, 5, and 10 mg/kg/day in males and 5, 10, and 25 mg/kg/day in females) for 2 years by oral gavage. The systemic drug exposure (AUC ng•h/mL) at the 10 mg/kg/day dose in male rats was 0.34-fold and at the 25 mg/kg/day dose in female rats was 1.5-fold the human exposure at a dose of 260 mg/day. No evidence of carcinogenic potential was observed in rats. Oxymorphone was administered to CD-1 mice (10, 25, 75 and 150 mg/kg/day) for 2 years by oral gavage. The systemic drug exposure (AUC ng•h/mL) at the 150 mg/kg/day dose in mice was 14.5-fold (in males) and 17.3-fold (in females) times the human exposure at a dose of 260 mg/day. No evidence of carcinogenic potential was observed in mice.
Mutagenesis
Oxymorphone hydrochloride was not mutagenic when tested in the in vitro bacterial reverse mutation assay (Ames test) at concentrations of ≤5270 µg/plate, or in an in vitro mammalian cell chromosome aberration assay performed with human peripheral blood lymphocytes at concentrations ≤5000 µg/ml with or without metabolic activation. Oxymorphone hydrochloride tested positive in both the rat and mouse in vivo micronucleus assays. An increase in micronucleated polychromatic erythrocytes occurred in mice given doses of ≥250 mg/kg and in rats given doses of 20 and 40 mg/kg. A subsequent study demonstrated that oxymorphone hydrochloride was not aneugenic in mice following administration of up to 500 mg/kg. Additional studies indicate that the increased incidence of micronucleated polychromatic erythrocytes in rats may be secondary to increased body temperature following oxymorphone administration. Doses associated with increased micronucleated polychromatic erythrocytes also produce a marked, rapid increase in body temperature. Pretreatment of animals with sodium salicylate minimized the increase in body temperature and prevented the increase in micronucleated polychromatic erythrocytes after administration of 40 mg/kg oxymorphone.
Impairment of fertility
Oxymorphone did not affect reproductive function or sperm parameters in male rats at any dose tested (≤50 mg/kg/day). In female rats, an increase in the length of the estrus cycle and decrease in the mean number of viable embryos, implantation sites and corpora lutea were observed at doses of oxymorphone ≥10 mg/kg/day. The dose of oxymorphone associated with reproductive findings in female rats is 0.8 times a total human daily dose of 120 mg based on a body surface area. The dose of oxymorphone that produced no adverse effects on reproductive findings in female rats (i.e., NOAEL) is 0.4-times a total human daily dose of 120 mg based on body surface area.
The analgesic efficacy of OPANA has been evaluated in acute pain following orthopedic and abdominal surgeries.
Two double-blind, placebo-controlled, dose-ranging studies in patients with acute moderate to severe pain following orthopedic surgery evaluated the doses of OPANA 10 mg and 20 mg, and 30 mg was included in one study. Both studies demonstrated that OPANA 20 mg provided greater analgesia as measured by total pain relief based on a weighted analysis over 8 hours using a 0-4 categorical, compared to placebo. OPANA 10 mg provided greater analgesia as compared to placebo in one of the two studies. There was no evidence of superiority of the 30 mg dose over the 20 mg dose. However, there was a high rate of naloxone use in patients receiving the OPANA 30 mg dose in the post-operative period [see Dosage and Administration (2.2)].
In a randomized, double-blind, placebo-controlled, multiple-dose study, the efficacy of OPANA 10 mg and 20 mg was assessed in patients with moderate to severe acute pain following abdominal surgery. In this study, patients were dosed every 4 to 6 hours over a 48-hour treatment period. OPANA 10 and 20 mg provided greater analgesia, as measured by the mean average pain intensity on a 0-100 mm visual analog scale, over 48 hours, compared to placebo [see Dosage and Administration (2.2)].
OPANA tablets are supplied as follows:
5 mg Tablet:
Blue, round, convex tablets debossed with E612 over 5 on one side and plain on the other.
Bottles of 100 tablets with child-resistant closure NDC 63481-612-70
Unit-Dose package of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for hospital use only) NDC 63481-612-75
10 mg Tablet:
Red, round, convex tablets debossed with E613 over 10 on one side and plain on the other.
Bottles of 100 tablets with child-resistant closure NDC 63481-613-70
Unit-Dose package of 100 tablets (5 blister cards of 20
tablets, not child-resistant, for hospital use only) NDC 63481-613-75
OPANA may be targeted for theft and diversion. Healthcare professionals should contact their State Medical Board, State Board of Pharmacy, or State Control Board for information on how to detect or prevent diversion of this product, and security requirements for storing and handling of OPANA.
Healthcare professionals should advise patients to store OPANA in a secure place, preferably locked and out of the reach of children and other non-caregivers.
Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F). [See USP Controlled Room Temperature].
Dispense in tight container as defined in the USP, with a child-resistant closure (as required).
Advise patients to dispose of any unused tablets from a prescription by flushing them down the toilet as soon as they are no longer needed [see Patient Counseling Information (17)].
17 PATIENT COUNSELING INFORMATION
- Advise patients that OPANA contains oxymorphone, which is a morphine-like pain reliever, and should be taken only as directed.
- Advise patients that OPANA is a potential drug of abuse. They should protect it from theft, and it should never be given to anyone other than the individual for whom it was prescribed.
- Advise patients to keep OPANA in a secure place out of the reach of children and pets. Accidental consumption especially in children may result in overdose or death. When OPANA is no longer needed, the unused tablets should be destroyed by flushing down the toilet.
- Advise patients to report episodes of breakthrough pain and adverse experiences occurring during therapy to their doctor. Individualization of dosage is essential to make optimal use of this medication.
- Advise patients not to adjust the dose of OPANA without consulting the prescriber.
- Advise patients that OPANA may cause drowsiness, dizziness, or lightheadedness and may impair mental and/or physical abilities required for the performance of potentially hazardous tasks, such as driving a car, operating machinery, etc.
- Advise patients that OPANA will add to the effect of alcohol and other CNS depressants (such as antihistamines, sedatives, hypnotics, tranquilizers, general anesthetics, phenothiazines, other opioids, and monoamine oxidase [MAO] inhibitors).
- Advise patients not to combine OPANA with alcohol or other central nervous system depressants (sleep aids, tranquilizers) except by the orders of the prescribing physician, because dangerous additive effects may occur, resulting in serious injury or death.
- Advise patients of the potential for severe constipation. Appropriate laxatives and/or stool softeners and other therapeutic approaches should be considered for use with the initiation of OPANA therapy.
- Advise women of childbearing potential who become or are planning to become pregnant to consult their physician regarding the effects of opioid analgesics and other drug use during pregnancy on themselves and their unborn child.
- Advise women of childbearing potential who become or are planning to become pregnant that safe use in pregnancy has not been established. Prolonged use of opioid analgesics during pregnancy may cause fetal-neonatal physical dependence, and neonatal withdrawal may occur.
- Advise patients that if they have been receiving treatment with OPANA for more than a few weeks and cessation of therapy is indicated, it may be appropriate to taper the OPANA dose, rather than abruptly discontinue it, due to the risk of precipitating withdrawal symptoms. Their physician can provide a dose schedule to accomplish a gradual discontinuation of the medication.
Manufactured for:
Endo Pharmaceuticals Inc.
Malvern, PA 19355
OPANA® is a registered trademark of Endo Pharmaceuticals Inc.
© 2013 Endo Pharmaceuticals Inc. All rights reserved.
8183490 R0
March 2013


OPANAOxymorphone Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
OPANAOxymorphone Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||