ONDANSETRON HYDROCHLORIDE
Bryant Ranch Prepack
Bryant Ranch Prepack
Ondansetron Hydrochloride Tablets
FULL PRESCRIBING INFORMATION: CONTENTS*
- ONDANSETRON HYDROCHLORIDE DESCRIPTION
- ONDANSETRON HYDROCHLORIDE INDICATIONS AND USAGE
- Ondansetron Hcl 4mg Tablet
FULL PRESCRIBING INFORMATION
ONDANSETRON HYDROCHLORIDE DESCRIPTION
The active ingredient in ondansetron tablets is ondansetron hydrochloride (HCl) as the dihydrate, the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula: 3
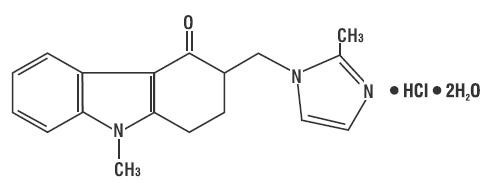
The empirical formula is C H N O•HCl•2H O, representing a molecular weight of 365.9. 18 19 3 2
Ondansetron HCl dihydrate is a white to off-white powder that is soluble in water and normal saline.
Each 4 mg ondansetron tablet for oral administration contains ondansetron hydrochloride dihydrate equivalent to 4 mg of ondansetron. Each 8 mg ondansetron tablet for oral administration contains ondansetron hydrochloride dihydrate equivalent to 8 mg of ondansetron. Each tablet also contains the inactive ingredients anhydrous lactose, microcrystalline cellulose, pregelatinized starch, hypromellose, magnesium stearate, titanium dioxide, and polyethylene glycol 400. Ondansetron tablet 8 mg also contains polysorbate 80 and iron oxide yellow.
Ondansetron is a selective 5-HT receptor antagonist. While its mechanism of action has not been fully characterized, ondansetron is not a dopamine-receptor antagonist. Serotonin receptors of the 5-HT type are present both peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. It is not certain whether ondansetron’s antiemetic action is mediated centrally, peripherally, or in both sites. However, cytotoxic chemotherapy appears to be associated with release of serotonin from the enterochromaffin cells of the small intestine. In humans, urinary 5-HIAA (5-hydroxyindoleacetic acid) excretion increases after cisplatin administration in parallel with the onset of emesis. The released serotonin may stimulate the vagal afferents through the 5-HT receptors and initiate the vomiting reflex. 3 3 3
In animals, the emetic response to cisplatin can be prevented by pretreatment with an inhibitor of serotonin synthesis, bilateral abdominal vagotomy and greater splanchnic nerve section, or pretreatment with a serotonin 5-HT receptor antagonist. 3
In normal volunteers, single intravenous doses of 0.15 mg/kg of ondansetron had no effect on esophageal motility, gastric motility, lower esophageal sphincter pressure, or small intestinal transit time. Multiday administration of ondansetron has been shown to slow colonic transit in normal volunteers. Ondansetron has no effect on plasma prolactin concentrations.
Ondansetron does not alter the respiratory depressant effects produced by alfentanil or the degree of neuromuscular blockade produced by atracurium. Interactions with general or local anesthetics have not been studied.
Ondansetron is well absorbed from the gastrointestinal tract and undergoes some first-pass metabolism. Mean bioavailability in healthy subjects, following administration of a single 8 mg tablet, is approximately 56%.
Ondansetron systemic exposure does not increase proportionately to dose. AUC from a 16 mg tablet was 24% greater than predicted from an 8 mg tablet dose. This may reflect some reduction of first-pass metabolism at higher oral doses. Bioavailability is also slightly enhanced by the presence of food but unaffected by antacids.
Ondansetron is extensively metabolized in humans, with approximately 5% of a radiolabeled dose recovered as the parent compound from the urine. The primary metabolic pathway is hydroxylation on the indole ring followed by subsequent glucuronide or sulfate conjugation. Although some nonconjugated metabolites have pharmacologic activity, these are not found in plasma at concentrations likely to significantly contribute to the biological activity of ondansetron.
In vitro metabolism studies have shown that ondansetron is a substrate for human hepatic cytochrome P-450 enzymes, including CYP1A2, CYP2D6, and CYP3A4. In terms of overall ondansetron turnover, CYP3A4 played the predominant role. Because of the multiplicity of metabolic enzymes capable of metabolizing ondansetron, it is likely that inhibition or loss of one enzyme (e.g., CYP2D6 genetic deficiency) will be compensated by others and may result in little change in overall rates of ondansetron elimination. Ondansetron elimination may be affected by cytochrome P-450 inducers. In a pharmacokinetic study of 16 epileptic patients maintained chronically on CYP3A4 inducers, carbamazepine, or phenytoin, reduction in AUC, C , and T of ondansetron was observed. This resulted in a significant increase in clearance. However, on the basis of available data, no dosage adjustment for is recommended (see ). max ½ 1 ondansetron PRECAUTIONS: Drug Interactions
In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron.
Gender differences were shown in the disposition of ondansetron given as a single dose. The extent and rate of ondansetron's absorption is greater in women than men. Slower clearance in women, a smaller apparent volume of distribution (adjusted for weight), and higher absolute bioavailability resulted in higher plasma ondansetron levels. These higher plasma levels may in part be explained by differences in body weight between men and women. It is not known whether these gender-related differences were clinically important. More detailed pharmacokinetic information is contained in Tables and taken from 2 studies. 1 2
|
Age-group (years) |
Mean Weight (kg) |
N |
Peak Plasma Concentration (ng/mL) |
Time of Peak Plasma Concentration (h) |
Mean Elimination Half-life (h) |
Systemic Plasma Clearance (L/h/kg) |
Absolute Bioavailability |
|
18-40 M F |
69.0 62.7 |
6 5 |
26.2 42.7 |
2.0 1.7 |
3.1 3.5 |
0.403 0.354 |
0.483 0.663 |
|
61-74 M F |
77.5 60.2 |
6 6 |
24.1 52.4 |
2.1 1.9 |
4.1 4.9 |
0.384 0.255 |
0.585 0.643 |
|
≥ 75 M F |
78.0 67.6 |
5 6 |
37.0 46.1 |
2.2 2.1 |
4.5 6.2 |
0.277 0.249 |
0.619 0.747 |
|
Age-group (years) |
Mean Weight (kg) |
n |
Peak Plasma Concentration (ng/mL) |
Time of Peak Plasma Concentration (h) |
Mean Elimination Half-life (h) |
|
18-43 M F |
84.1 71.8 |
8 8 |
125.8 194.4 |
1.9 1.6 |
4.7 5.8 |
A reduction in clearance and increase in elimination half-life are seen in patients over 75 years of age. In clinical trials with cancer patients, safety and efficacy was similar in patients over 65 years of age and those under 65 years of age; there was an insufficient number of patients over 75 years of age to permit conclusions in that age-group. No dosage adjustment is recommended in the elderly.
In patients with mild-to-moderate hepatic impairment, clearance is reduced 2-fold and mean half-life is increased to 11.6 hours compared to 5.7 hours in normals. In patients with severe hepatic impairment (Child-Pugh score of 10 or greater), clearance is reduced 2-fold to 3-fold and apparent volume of distribution is increased with a resultant increase in half-life to 20 hours. In patients with severe hepatic impairment, a total daily dose of 8 mg should not be exceeded. 2
Due to the very small contribution (5%) of renal clearance to the overall clearance, renal impairment was not expected to significantly influence the total clearance of ondansetron. However, ondansetron oral mean plasma clearance was reduced by about 50% in patients with severe renal impairment (creatinine clearance <30 mL/min). This reduction in clearance is variable and was not consistent with an increase in half-life. No reduction in dose or dosing frequency in these patients is warranted.
Plasma protein binding of ondansetron as measured in vitro was 70% to 76% over the concentration range of 10 to 500 ng/mL. Circulating drug also distributes into erythrocytes.
One 24 mg ondansetron tablet is bioequivalent to and interchangeable with three 8 mg ondansetron tablets.
ONDANSETRON HYDROCHLORIDE INDICATIONS AND USAGE
1. Prevention of nausea and vomiting associated with highly emetogenic cancer chemotherapy, including cisplatin ≥50 mg/m . 2
2. Prevention of nausea and vomiting associated with initial and repeat course of moderately emetogenic cancer chemotherapy.
3. Prevention of nausea and vomiting associated with radiotherapy in patients receiving either total body irradiation, single high-dose fraction to the abdomen, or daily fractions to the abdomen.
4. Prevention of postoperative nausea and/or vomiting. As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron tablets are recommended even where the incidence of postoperative nausea and/or vomiting is low.
Ondansetron tablets are contraindicated for patients known to have hypersensitivity to the drug.
Hypersensitivity reactions have been reported in patient who have exhibited hypersensitivity to other selective 5-HT receptor antagonists. 3
Ondansetron is not a drug that stimulates gastric or intestinal peristalsis. It should not be used instead of nasogastric suction. The use of ondansetron in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distension.
Rarely and predominantly with intravenous ondansetron, transient ECG changes including QT interval prolongation have been reported.
Ondansetron does not itself appear to induce or inhibit the cytochrome P-450 drug-metabolizing enzyme system of the liver (see ). Because ondansetron is metabolized by hepatic cytochrome P-450 drug-metabolizing enzymes (CYP3A4, CYP2D6, CYP1A2), inducers or inhibitors of these enzymes may change the clearance and, hence, the half-life of ondansetron. On the basis of available data, no dosage adjustment is recommended for patients on these drugs. CLINICAL PHARMACOLOGY, Pharmacokinetics
In patients treated with potent inducers of CYP3A4 (i.e., phenytoin, carbamazepine, and rifampicin), the clearance of ondansetron was significantly increased and ondansetron blood concentrations were decreased. However, on the basis of available data, no dosage adjustment for ondansetron is recommended for patients on these drugs. Phenytoin, Carbamazepine, and Rifampicin: 1,3
Although no pharmacokinetic drug interaction between ondansetron and tramadol has been observed, data from 2 small studies indicate that ondansetron may be associated with an increase in patient controlled administration of tramadol. Tramadol: 4,5
Tumor response to chemotherapy in the P-388 mouse leukemia model is not affected by ondansetron. In humans, carmustine, etoposide, and cisplatin do not affect the pharmacokinetics of ondansetron. Chemotherapy:
In a crossover study in 76 pediatric patients, I.V. ondansetron did not increase blood levels of high-dose methotrexate.
Carcinogenic effects were not seen in 2-year studies in rats and mice with oral ondansetron doses up to 10 and 30 mg/kg per day, respectively. Ondansetron was not mutagenic in standard tests for mutagenicity. Oral administration of ondansetron up to 15 mg/kg per day did not affect fertility or general reproductive performance of male and female rats.
Pregnancy Category B. Reproduction studies have been performed in pregnant rats and rabbits at daily oral doses up to 15 and 30 mg/kg per day, respectively, and have revealed no evidence of impaired fertility or harm to the fetus due to ondansetron. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed. Teratogenic Effects :
Ondansetron is excreted in the breast milk of rats. It is not known whether ondansetron is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when ondansetron is administered to a nursing woman.
Little information is available about dosage in pediatric patients 4 years of age or younger (see sections for use in pediatric patients 4 to 18 years of age). CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION
Of the total number of subjects enrolled in cancer chemotherapy-induced and postoperative nausea and vomiting in US- and foreign-controlled clinical trials, for which there were subgroup analyses, 938 were 65 years of age and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. Dosage adjustment is not needed in patients over the age of 65 (see ). CLINICAL PHARMACOLOGY
The following have been reported as adverse events in clinical trials of patients treated with ondansetron. A causal relationship to therapy with ondansetron has been unclear in many cases.
Animal studies have shown that ondansetron is not discriminated as a benzodiazepine nor does it substitute for benzodiazepines in direct addiction studies.
There is no specific antidote for ondansetron overdose. Patients should be managed with appropriate supportive therapy. Individual intravenous doses as large as 150 mg and total daily intravenous doses as large as 252 mg have been inadvertently administered without significant adverse events. These doses are more than 10 times the recommended daily dose.
In addition to the adverse events listed above, the following events have been described in the setting of ondansetron overdose: "Sudden blindness" (amaurosis) of 2 to 3 minutes duration plus severe constipation occurred in one patient that was administered 72 mg of ondansetron intravenously as a single dose. Hypotension (and faintness) occurred in a patient that took 48 mg of ondansetron tablets. Following infusion of 32 mg over only a 4-minute period, a vasovagal episode with transient second-degree heart block was observed. In all instances, the events resolved completely.
1. Britto MR, Hussey EK, Mydlow P, et al. Effect of enzyme inducers on ondansetron (OND) metabolism in humans. 1997;61:228. Clin Pharmacol Ther
2. Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. 1973;60:646-649. Brit J Surg
3. Villikka K, Kivisto KT, Neuvonen PJ. The effect of rifampin on the pharmacokinetics of oral and intravenous ondansetron. Clin Pharmacol Ther 1999;65:377-381.
4. De Witte JL, Schoenmaekers B, Sessler DI, et al. 2001;92:1319-1321. Anesth Analg
5. Arcioni R, della Rocca M, Romanò R, et al. 2002;94:1553-1557. Anesth Analg
Ondansetron Hcl 4mg Tablet

ONDANSETRON HYDROCHLORIDEONDANSETRON HYDROCHLORIDE TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||