Ondansetron Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- ONDANSETRON HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACODYNAMICS
- PHARMACOKINETICS
- INDICATIONS & USAGE
- ONDANSETRON HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- ONDANSETRON HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
ONDANSETRON HYDROCHLORIDE DESCRIPTION
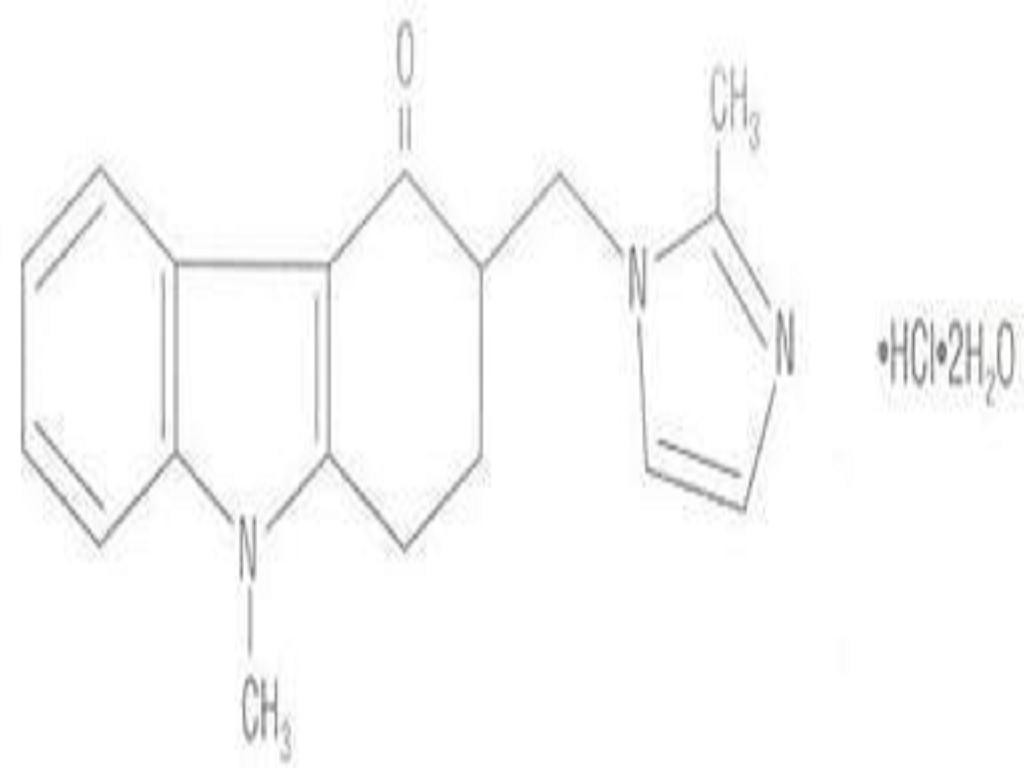
CLINICAL PHARMACOLOGY
PHARMACODYNAMICS
PHARMACOKINETICS
PRECAUTIONS: Drug Interactions
Age-group (years)Mean Weight (kg)nMean Peak Plasma Concentration (ng/mL)Time of Peak Plasma Concentration (h)Mean Elimination Half-life (h)Systemic Plasma Clearance L/h/kgAbsolute Bioavailability
Age-group (years)Mean Weight (kg)nPeak Plasma Concentration (ng/mL)Time of Peak Plasma Concentration (h)Mean Elimination Half-life (h)
CLINICAL TRIALS
Ondansetron 8-mg b.i.d. Ondansetron Hydrochloride Tablets *Placebop ValueNumber of patients3334
INDICATIONS & USAGE
ONDANSETRON HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
PRECAUTIONS
DRUG INTERACTIONS
CLINICAL PHARMACOLOGY, PharmacokineticsCARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATIONGERIATRIC USE
CLINICAL PHARMACOLOGYONDANSETRON HYDROCHLORIDE ADVERSE REACTIONS
EventOndansetron 24 mg q.d n = 300Ondansetron 8 mg b.i.d. n = 124Ondansetron 32 mg q.d. n = 117
EventOndansetron 8 mg b.i.d. n = 242Ondansetron 8 mg t.i.d. n = 415Placebo n = 262
Adverse EventOndansetron 16 mg (n = 550)Placebo (n = 531)
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
DOSAGE & ADMINISTRATION
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Ondansetron HydrochlorideOndansetron Hydrochloride TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!