ONDANSETRON HYDROCHLORIDE
PRESCRIBING INFORMATION ONDANSETRON INJECTION USP
FULL PRESCRIBING INFORMATION: CONTENTS*
- ONDANSETRON HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL TRIALS
- ONDANSETRON HYDROCHLORIDE INDICATIONS AND USAGE
- ONDANSETRON HYDROCHLORIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- ONDANSETRON HYDROCHLORIDE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- ONDANSETRON HYDROCHLORIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
FULL PRESCRIBING INFORMATION
ONDANSETRON HYDROCHLORIDE DESCRIPTION
The active ingredient in Ondansetron Injection is ondansetron hydrochloride (HCl), the racemic form of ondansetron and a selective blocking agent of the serotonin 5-HT3 receptor type. Chemically it is (±) 1, 2, 3, 9-tetrahydro-9-methyl-3-[(2-methyl-1H-imidazol-1-yl)methyl]-4H-carbazol-4-one, monohydrochloride, dihydrate. It has the following structural formula:
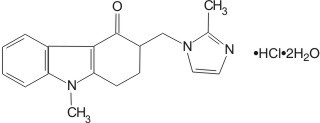
The empirical formula is C18H19N3O•HCl•2H2O, representing a molecular weight of 365.9.
Ondansetron HCl is a white to off-white powder that is soluble in water and normal saline.
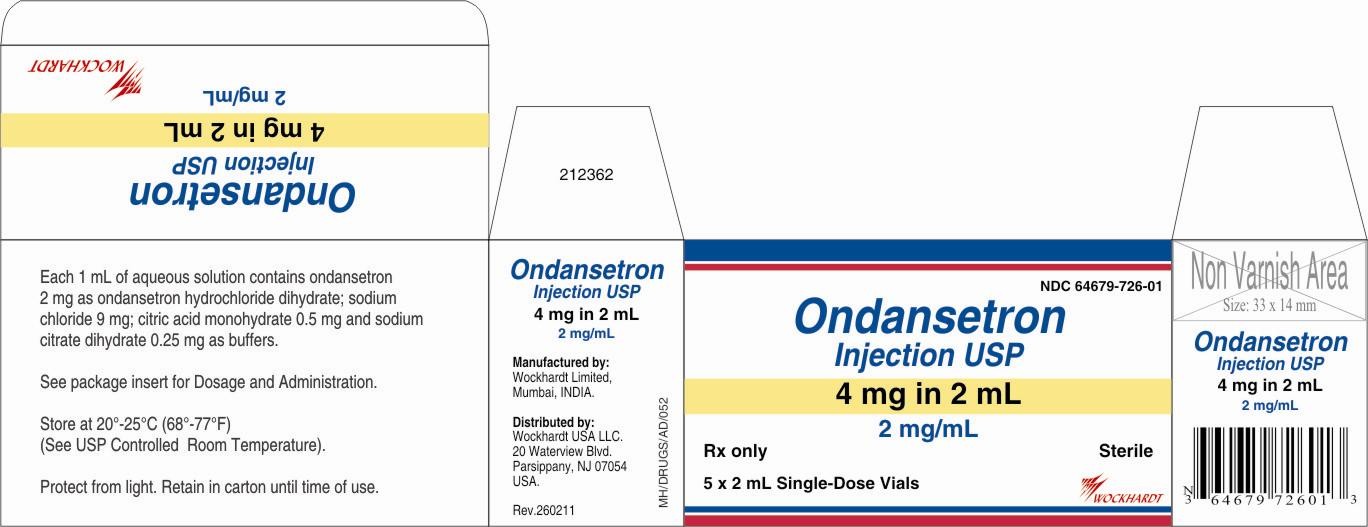
Sterile Injection for Intravenous (I.V.) or Intramuscular (I.M.) Administration: Each 1 mL of aqueous solution in the 2-mL single-dose vial contains 2 mg of ondansetron as the hydrochloride dihydrate; 9 mg of sodium chloride, USP; and 0.5 mg of citric acid monohydrate, USP and 0.25 mg of sodium citrate dihydrate, USP as buffers in Water for Injection, USP.
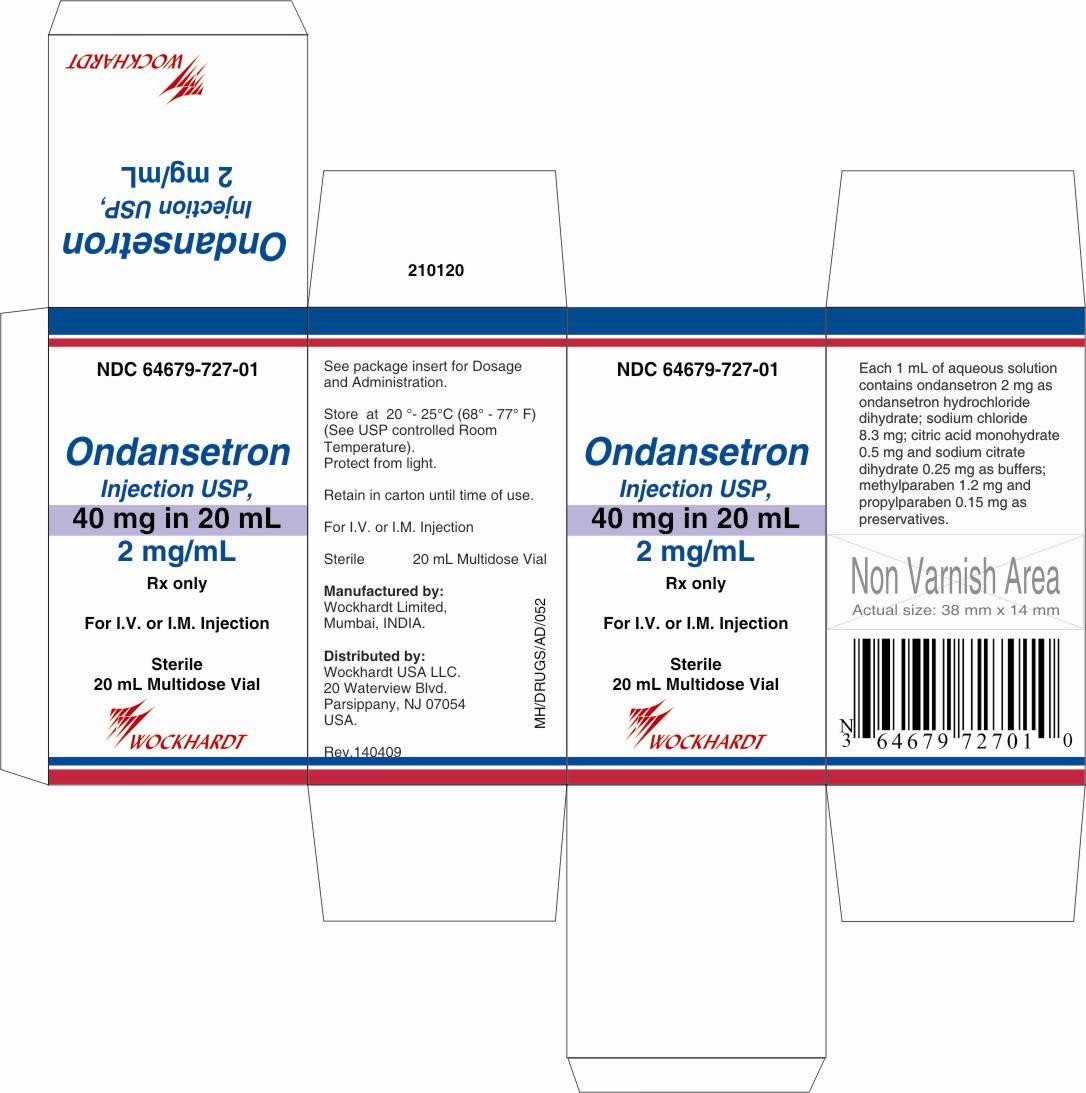
Each 1 mL of aqueous solution in the 20-mL multidose vial contains 2 mg of ondansetron as the hydrochloride dihydrate; 8.3 mg of sodium chloride, USP; 0.5 mg of citric acid monohydrate, USP and 0.25 mg of sodium citrate dihydrate, USP as buffers; and 1.2 mg of methylparaben, NF and 0.15 mg of propylparaben, NF as preservatives in Water for Injection, USP.
Ondansetron Injection is a clear, colorless, nonpyrogenic, sterile solution. The pH of the injection solution is 3.3 to 4.0.
CLINICAL PHARMACOLOGY
Pharmacodynamics:3333
Pharmacokinetics:
In vitro max1
|
Age-group (years) |
n |
Peak Plasma
Concentration (ng/mL) |
Mean Elimination Half-life (h) |
Plasma Clearance (L/h/kg) |
| 19-40 | 11 | 102 | 3.5 | 0.381 |
| 61-74 | 12 | 106 | 4.7 | 0.319 |
| ≥75 | 11 | 170 | 5.5 | 0.262 |
2
|
Subjects and Age Group |
N |
CL (L/h/kg) |
Vdss (L/kg) |
T½ (h) |
| Geometric Mean | Mean | |||
|
Pediatric Cancer Patients 4 to 18 years of age |
N = 21 | 0.599 | 1.9 | 2.8 |
|
Population PK Patientsa 1 month to 48 months of age |
N = 115 | 0.582 | 3.65 | 4.9 |
| Subjects and Age Group |
N |
CL (L/h/kg) |
Vdss
(L/kg) |
T½
(h) |
| Geometric Mean | Mean | |||
|
Pediatric Surgery Patients 3 to 12 years of Age |
N = 21 | 0.439 | 1.65 | 2.9 |
|
Pediatric Surgery Patients 5 to 24 months of Age |
N = 22 | 0.581 | 2.3 | 2.9 |
|
Pediatric Surgery Patients 1 month to 4 months of Age |
N = 19 | 0.401 | 3.5 | 6.7 |
in vitro
CLINICAL TRIALS
Chemotherapy-Induced Nausea and Vomiting: Adult Studies:Cisplatin-Based Chemotherapy:
| Ondansetron Injection | Placebo | P Value† | |
| Number of patients | 14 | 14 | |
| Treatment response | |||
| 0 Emetic episodes | 2 (14%) | 0 (0%) | |
| 1-2 Emetic episodes | 8 (57%) | 0 (0%) | |
| 3-5 Emetic episodes | 2 (14%) | 1 (7%) | |
| More than 5 emetic episodes/rescued | 2 (14%) | 13 (93%) | 0.001 |
| Median number of emetic episodes | 1.5 | Undefined‡ | |
| Median time to first emetic episode (h) | 11.6 | 2.8 | 0.001 |
| Median nausea scores (0-100)§ | 3 | 59 | 0.034 |
| Global satisfaction with control of nausea and vomiting (0-100)II | 96 | 10.5 | 0.009 |
†
‡
§
II
2
| Ondansetron Injection | Metoclopramide | P Value | |
| Dose | 0.15 mg/kg x 3 | 2 mg/kg x 6 | |
| Number of patients in efficacy population | 136 | 138 | |
| Treatment response | |||
| 0 Emetic episodes | 54 (40%) | 41 (30%) | |
| 1-2 Emetic episodes | 34 (25%) | 30 (22%) | |
| 3-5 Emetic episodes | 19 (14%) | 18 (13%) | |
| More than 5 emetic episodes/rescued | 29 (21%) | 49 (36%) | |
| Comparison of treatments with respect to | |||
| 0 Emetic episodes | 54/136 | 41/138 | 0.083 |
| More than 5 emetic episodes/rescued | 29/136 | 49/138 | 0.009 |
| Median number of emetic episodes | 1 | 2 | 0.005 |
| Median time to first emetic episode (h) | 20.5 | 4.3 | <0.001 |
| Global satisfaction with control of nausea and vomiting (0-100)† | 85 | 63 | 0.001 |
| Acute dystonic reactions | 0 | 8 | 0.005 |
| Akathisia | 0 | 10 | 0.002 |
†
22
|
Ondansetron Dose |
|||
| 0.15 mg/kg x 3 | 32 mg x 1 | P Value | |
| High-dose cisplatin (≥100 mg/m2) | |||
| Number of patients | 100 | 102 | |
| Treatment response | |||
| 0 Emetic episodes | 41 (41%) | 49 (48%) | 0.315 |
| 1-2 Emetic episodes | 19 (19%) | 25 (25%) | |
| 3-5 Emetic episodes | 4 (4%) | 8 (8%) | |
| More than 5 emetic episodes/rescued | 36 (36%) | 20 (20%) | 0.009 |
| Median time to first emetic episode (h) | 21.7 | 23 | 0.173 |
| Median nausea scores (0-100)* | 28 | 13 | 0.004 |
| Medium-dose cisplatin (50-70 mg/m2) | |||
| Number of patients | 101 | 93 | |
| Treatment response | |||
| 0 Emetic episodes | 62 (61%) | 68 (73%) | 0.083 |
| 1-2 Emetic episodes | 11 (11%) | 14 (15%) | |
| 3-5 Emetic episodes | 6 (6%) | 3 (3%) | |
| More than 5 emetic episodes/rescued | 22 (22%) | 8 (9%) | 0.011 |
| Median time to first emetic episode (h) | Undefined† | Undefined | |
| Median nausea scores (0-100)* | 9 | 3 | 0.131 |
†
Cyclophosphamide-Based Chemotherapy: 2
| Ondansetron Injection | Placebo | P Value† | |
| Number of patients | 10 | 10 | |
| Treatment response | |||
| 0 Emetic episodes | 7 (70%) | 0 (0%) | 0.001 |
| 1-2 Emetic episodes | 0 (0%) | 2 (20%) | |
| 3-5 Emetic episodes | 2 (20%) | 4 (40%) | |
| More than 5 emetic episodes/rescued | 1 (10%) | 4 (40%) | 0.131 |
| Median number of emetic episodes | 0 | 4 | 0.008 |
| Median time to first emetic episode (h) | Undefined‡ | 8.79 | |
| Median nausea scores (0-100)§ | 0 | 60 | 0.001 |
| Global satisfaction with control of nausea and vomiting (0-100)II | 100 | 52 | 0.008 |
†
‡
§
II
Re-treatment: 2
Pediatric Studies:
Postoperative Nausea and Vomiting: Prevention of Postoperative Nausea and Vomiting: Adult Studies:
| Ondansetron 4 mg I.V. | Placebo | P Value | |
| Study 1 | |||
| Emetic episodes: | |||
| Number of patients | 136 | 139 | |
| Treatment response over 24-h postoperative period | |||
| 0 Emetic episodes | 103 (76%) | 64 (46%) | <0.001 |
| 1 Emetic episode | 13 (10%) | 17 (12%) | |
| More than 1 emetic episode/rescued | 20 (15%) | 58 (42%) | |
| Nausea assessments: | |||
| Number of patients | 134 | 136 | |
| No nausea over 24-h postoperative period | 56 (42%) | 39 (29%) | |
| Study 2 | |||
| Emetic episodes: | |||
| Number of patients | 136 | 143 | |
| Treatment response over 24-h postoperative period | |||
| 0 Emetic episodes | 85 (63%) | 63 (44%) | 0.002 |
| 1 Emetic episode | 16 (12%) | 29 (20%) | |
| More than 1 emetic episode/rescued | 35 (26%) | 51 (36%) | |
| Nausea assessments: | |||
| Number of patients | 125 | 133 | |
| No nausea over 24-h postoperative period | 48 (38%) | 42 (32%) |
P
PP
Pediatric Studies:
|
Treatment Response Over 24 Hours |
Ondansetron n (%) |
Placebo n (%) |
P Value |
| Study 1 | |||
| Number of patients | 205 | 210 | |
| 0 Emetic episodes | 140 (68%) | 82 (39%) | ≤0.001 |
| Failure* | 65 (32%) | 128 (61%) | |
| Study 2 | |||
| Number of patients | 112 | 110 | |
| 0 Emetic episodes | 68 (61%) | 38 (35%) | ≤0.001 |
| Failure* | 44 (39%) | 72 (65%) | |
| Study 3 | |||
| Number of patients | 206 | 206 | |
| 0 Emetic episodes | 123 (60%) | 96 (47%) | ≤0.01 |
| Failure* | 83 (40%) | 110 (53%) | |
| Nausea assessments†: | |||
| Number of patients | 185 | 191 | |
| None | 119 (64%) | 99 (52%) | ≤0.01 |
†
P
Prevention of Further Postoperative Nausea and Vomiting: Adult Studies:
|
Ondansetron 4 mg I.V. |
Placebo | P Value | |
| Study 1 | |||
| Emetic episodes: | |||
| Number of patients | 104 | 117 | |
| Treatment response 24 h after study drug | |||
| 0 Emetic episodes | 49 (47%) | 19 (16%) | <0.001 |
| 1 Emetic episode | 12 (12%) | 9 (8%) | |
| More than 1 emetic episode/rescued | 43 (41%) | 89 (76%) | |
| Median time to first emetic episode (min)* | 55.0 | 43.0 | |
| Nausea assessments: | |||
| Number of patients | 98 | 102 | |
| Mean nausea score over 24-hpostoperative period† | 1.7 | 3.1 | |
| Study 2 | |||
| Emetic episodes: | |||
| Number of patients | 112 | 108 | |
| Treatment response 24 h after study drug | |||
| 0 Emetic episodes | 49 (44%) | 28 (26%) | 0.006 |
| 1 Emetic episode | 14 (13%) | 3 (3%) | |
| More than 1 emetic episode/rescued | 49 (44%) | 77 (71%) | |
| Median time to first emetic episode (min)* | 60.5 | 34.0 | |
| Nausea assessments: | |||
| Number of patients | 105 | 85 | |
| Mean nausea score over 24-hpostoperative period† | 1.9 | 2.9 |
†
Repeat Dosing in Adults:
Pediatric Study:
|
Treatment Response Over 24 Hours |
Ondansetron n (%) |
Placebo n (%) |
P Value |
| Number of patients | 180 | 171 | |
| 0 Emetic episodes | 96 (53%) | 29 (17%) | ≤0.001 |
| Failure* | 84 (47%) | 142 (83%) |
ONDANSETRON HYDROCHLORIDE INDICATIONS AND USAGE
- Prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including high-dose cisplatin. Efficacy of the 32-mg single dose beyond 24 hours in these patients has not been established.
- Prevention of postoperative nausea and/or vomiting. As with other antiemetics, routine prophylaxis is not recommended for patients in whom there is little expectation that nausea and/or vomiting will occur postoperatively. In patients where nausea and/or vomiting must be avoided postoperatively, ondansetron injection is recommended even where the incidence of postoperative nausea and/or vomiting is low. For patients who do not receive prophylactic ondansetron injection and experience nausea and/or vomiting postoperatively, ondansetron injection may be given to prevent further episodes (see CLINICAL TRIALS).
ONDANSETRON HYDROCHLORIDE CONTRAINDICATIONS
WARNINGS
3PRECAUTIONS
General:Drug Interactions:
Apomorphine:
Phenytoin, Carbamazepine, and Rifampicin: 1,3
Tramadol: 4,5
Chemotherapy:
Carcinogenesis, Mutagenesis, Impairment of Fertility:
Pregnancy: Teratogenic Effects:
Nursing Mothers:
Pediatric Use:
Geriatric Use:
ONDANSETRON HYDROCHLORIDE ADVERSE REACTIONS
Chemotherapy-Induced Nausea and Vomiting:| Number of Adult Patients With Event | ||||
|
Ondansetron Injection 0.15 mg/kg x 3 n = 419 |
Ondansetron Injection 32 mg x 1 n = 220 |
Metoclopramide n = 156 |
Placebo n = 34 |
|
| Diarrhea | 16% | 8% | 44% | 18% |
| Headache | 17% | 25% | 7% | 15% |
| Fever | 8% | 7% | 5% | 3% |
| Akathisia | 0% | 0% | 6% | 0% |
| Acute dystonic reactions* | 0% | 0% | 5% | 0% |
Cardiovascular:
Gastrointestinal:
Hepatic:
Integumentary:
Neurological:
Other:
Postoperative Nausea and Vomiting:
|
Ondansetron Injection 4 mg I.V. n = 547 patients |
Placebo n = 547 patients |
|
| Headache | 92 (17%) | 77 (14%) |
| Dizziness | 67 (12%) | 88 (16%) |
| Musculoskeletal pain | 57 (10%) | 59 (11%) |
| Drowsiness/sedation | 44 (8%) | 37 (7%) |
| Shivers | 38 (7%) | 39 (7%) |
| Malaise/fatigue | 25 (5%) | 30 (5%) |
| Injection site reaction | 21 (4%) | 18 (3%) |
| Urinary retention | 17 (3%) | 15 (3%) |
| Postoperative CO2-related pain* | 12 (2%) | 16 (3%) |
| Chest pain (unspecified) | 12 (2%) | 15 (3%) |
| Anxiety/agitation | 11 (2%) | 16 (3%) |
| Dysuria | 11 (2%) | 9 (2%) |
| Hypotension | 10 (2%) | 12 (2%) |
| Fever | 10 (2%) | 6 (1%) |
| Cold sensation | 9 (2%) | 8 (1%) |
| Pruritus | 9 (2%) | 3 (<1%) |
| Paresthesia | 9 (2%) | 2 (<1%) |
Pediatric Use:
|
Adverse Event |
Ondansetron n = 755 Patients |
Placebo n = 731 Patients |
| Wound problem | 80 (11%) | 86 (12%) |
| Anxiety/agitation | 49 (6%) | 47 (6%) |
| Headache | 44 (6%) | 43 (6%) |
| Drowsiness/sedation | 41 (5%) | 56 (8%) |
| Pyrexia | 32 (4%) | 41 (6%) |
|
Adverse Event |
Ondansetron n = 336 Patients |
Placebo n = 334 Patients |
| Pyrexia | 14 (4%) | 14 (4%) |
| Bronchospasm | 2 (< 1%) | 6 (2%) |
| Post-procedural pain | 4 (1%) | 6 (2%) |
| Diarrhea | 6 (2%) | 3 (< 1%) |
Cardiovascular:
General:
Hepatobiliary:
Local Reactions:
Lower Respiratory:
Neurological:
Skin:
Special Senses:
Eye Disorders:
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
ONDANSETRON HYDROCHLORIDE DOSAGE AND ADMINISTRATION
Prevention of Chemotherapy-Induced Nausea and Vomiting: Adult Dosing:Vial: DILUTE BEFORE USE FOR PREVENTION OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING.
Pediatric Dosing:
Vial: DILUTE BEFORE USE FOR PREVENTION OF CHEMOTHERAPY-INDUCED NAUSEA AND VOMITING.
Geriatric Dosing:
Prevention of Postoperative Nausea and Vomiting: Adult Dosing: undilutedundiluted
Vial: REQUIRES NO DILUTION FOR ADMINISTRATION FOR POSTOPERATIVE NAUSEA AND VOMITING.
Pediatric Dosing:
Vial: REQUIRES NO DILUTION FOR ADMINISTRATION FOR POSTOPERATIVE NAUSEA AND VOMITING.
Geriatric Dosing:
Dosage Adjustment for Patients With Impaired Renal Function:
Dosage Adjustment for Patients With Impaired Hepatic Function:
Stability:
Note:
Precaution:
HOW SUPPLIED
Ondansetron Injection,Store at 20°-25°C (68°-77°F) (See USP Controlled Room Temperature). Protect from light.
Retain in carton until time of use.
REFERENCES
- Britto MR, Hussey EK, Mydlow P, et al. Effect of enzyme inducers on ondansetron (OND) metabolism in humans. Clin Pharmacol Ther. 1997;61:228.
- Pugh RNH, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Brit J Surg. 1973;60:646-649.
- Villikka K, Kivisto KT, Neuvonen PJ. The effect of rifampin on the pharmacokinetics of oral and intravenous ondansetron. Clin Pharmacol Ther. 1999;65:377-381.
- De Witte JL, Schoenmaekers B, Sessler DI, et al. Anesth Analg. 2001;92:1319-1321.
- Arcioni R, della Rocca M, Romanò R, et al. Anesth Analg. 2002;94:1553-1557.
Manufactured by:
Distributed by:


ONDANSETRON HYDROCHLORIDEONDANSETRON HYDROCHLORIDE INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
ONDANSETRON HYDROCHLORIDEONDANSETRON HYDROCHLORIDE INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||