Ondansetron
HIGHLIGHTS OF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use ondansetron injection safely and effectively. See full prescribing information for ondansetron injection. ONDANSETRON INJECTION, USP for intravenous or intramuscular useInitial U.S. Approval: 1991RECENT MAJOR CHANGES2.15.2INDICATIONS AND USAGE3 Prevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy. (1.1) Prevention of postoperative nausea and/or vomiting. (1.2) DOSAGE AND ADMINISTRATIONPrevention of nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy (2.1): Adults and Pediatric patients (6 months to 18 years): Three 0.15 mg/kg doses, up to a maximum of 16 mg per dose, infused intravenously over 15 minutes. The first dose should be administered 30 minutes before the start of chemotherapy. Subsequent doses are administered 4 and 8 hours after the first dose. Prevention of postoperative nausea and/or vomiting (2.2): Population Age Ondansetron Injection Dosage Intravenous Infusion Rate Adults > 12 yrs 4 mg x 1 over 2 to 5 min Pediatrics(> 40 kg) 1 mo. to 12 yrs 4 mg x 1 over 2 to 5 min Pediatrics(≤ 40 kg) 1 mo. to 12 yrs 0.1 mg/kg x 1 over 2 to 5 min In patients with severe hepatic impairment, a total daily dose of 8 mg should not be exceeded. (2.4) DOSAGE FORMS AND STRENGTHSOndansetron injection (2 mg/mL): 2 mL single dose vial. (3)CONTRAINDICATIONS Patients known to have hypersensitivity (e.g., anaphylaxis) to this product or any of its components. (4) Concomitant use of apomorphine. (4) WARNINGS AND PRECAUTIONS Hypersensitivity reactions including anaphylaxis and bronchospasm, have been reported in patients who have exhibited hypersensitivity to other selective 5-HT3 receptor antagonists. (5.1) QT prolongation occurs in a dose-dependent manner. Cases of Torsade de Pointes have been reported. Avoid ondansetron in patients with congenital long QT syndrome. (5.2) Use in patients following abdominal surgery or in patients with chemotherapy-induced nausea and vomiting may mask a progressive ileus and/or gastric distention. (5.3)(5.4) Side Effects The most common adverse reactions (≥ 7%) in adults are diarrhea, headache, and fever. (6.1) Postoperative Nausea and Vomiting – The most common adverse reaction (≥ 10%) which occurs at a higher frequency compared to placebo in adults is headache. (6.1) The most common adverse reaction (≥ 2%) which occurs at a higher frequency compared to placebo in pediatric patients 1 to 24 months of age is diarrhea. (6.1) To report SUSPECTED ADVERSE REACTIONS, contact AuroMedics Pharma LLC at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch DRUG INTERACTIONS Apomorphine – profound hypotension and loss of consciousness. Concomitant use with ondansetron is contraindicated. (7.2)
FULL PRESCRIBING INFORMATION: CONTENTS*
- 1 ONDANSETRON INDICATIONS AND USAGE
- 2 ONDANSETRON DOSAGE AND ADMINISTRATION
- 3 DOSAGE FORMS AND STRENGTHS
- 4 ONDANSETRON CONTRAINDICATIONS
- 5 WARNINGS AND PRECAUTIONS
- 6 ONDANSETRON ADVERSE REACTIONS
- 7 DRUG INTERACTIONS
- 8 USE IN SPECIFIC POPULATIONS
- 9 DRUG ABUSE AND DEPENDENCE
- 10 OVERDOSAGE
- 11 ONDANSETRON DESCRIPTION
- 12 CLINICAL PHARMACOLOGY
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
- 17 PATIENT COUNSELING INFORMATION
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg/2 mL Container Label
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg/2 mL Container-Carton (25 Vials)
FULL PRESCRIBING INFORMATION
1 INDICATIONS AND USAGE
1.1 Prevention of Nausea and Vomiting Associated with Initial and Repeat Courses of Emetogenic Cancer Chemotherapy
[see Clinical Studies (14.1)].
1.2 Prevention of Postoperative Nausea and/or Vomiting
[see Clinical Studies (14.3)].
2 DOSAGE AND ADMINISTRATION
2.1 Prevention of Nausea and Vomiting Associated with Initial and Repeat Courses of Emetogenic Chemotherapy
Adults: The recommended adult intravenous dosage of ondansetron injection is three 0.15 mg/kg doses up to a maximum of 16 mg per dose [see Clinical Pharmacology (12.2)]. The first dose is infused over 15 minutes beginning 30 minutes before the start of emetogenic chemotherapy. Subsequent doses (0.15 mg/kg up to a maximum of 16 mg per dose) are administered 4 and 8 hours after the first dose of ondansetron injection.
Pediatrics: For pediatric patients 6 months through 18 years of age, the intravenous dosage of ondansetron injection is three 0.15 mg/kg doses up to a maximum of 16 mg per dose [see Clinical Studies (14.1) and Clinical Pharmacology (12.2 and 12.3)]. The first dose is to be administered 30 minutes before the start of moderately to highly emetogenic chemotherapy. Subsequent doses (0.15 mg/kg up to a maximum of 16 mg per dose) are administered 4 and 8 hours after the first dose of ondansetron injection. The drug should be infused intravenously over 15 minutes.
2.2 Prevention of Postoperative Nausea and Vomiting
Adults:undilutedundiluted
Pediatrics:
2.3 Stability and Handling
Note:
Precaution:
2.4 Dosage Adjustment for Patients with Impaired Hepatic Function
[see Clinical Pharmacology (12.3)].
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
[See Adverse Reactions (6.2).]
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
3
5.2 QT Prolongation
Ondansetron prolongs the QT interval in a dose-dependent manner [see Clinical Pharmacology (12.2)]. In addition, post-marketing cases of Torsade de Pointes have been reported in patients using ondansetron. Avoid ondansetron in patients with congenital long QT syndrome. ECG monitoring is recommended in patients with electrolyte abnormalities (e.g., hypokalemia or hypomagnesemia), congestive heart failure, bradyarrhythmias, or patients taking other medicinal products that lead to QT prolongation.
5.3 Masking of Progressive Ileus and Gastric Distension
5.4 Effect on Peristalsis
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Chemotherapy-Induced Nausea and Vomiting:
|
Adverse Reaction
|
Number of Adult Patients With Reaction
|
||
|
Ondansetron Injection
0.15 mg/kg x 3 n = 419 |
Metoclopramide
n = 156 |
Placebo
n = 34 |
|
| Diarrhea |
16% |
44% |
18% |
| Headache |
17% |
7% |
15% |
| Fever |
8% |
5% |
3% |
Cardiovascular:
Gastrointestinal:
Hepatic:
Integumentary:
Neurological:
Other:
Postoperative Nausea and Vomiting:
| Adverse Reactiona,b | Ondansetron Injection 4 mg Intravenous n = 547 patients |
Placebo n = 547 patients |
|---|---|---|
|
a Adverse Reactions: Rates of these reactions were not significantly different in the ondansetron and placebo groups b Patients were receiving multiple concomitant perioperative and postoperative medications |
||
| Headache |
92 (17%) |
77 (14%) |
| Drowsiness/sedation |
44 (8%) |
37 (7%) |
| Injection site reaction |
21 (4%) |
18 (3%) |
| Fever |
10 (2%) |
6 (1%) |
| Cold sensation |
9 (2%) |
8 (1%) |
| Pruritus |
9 (2%) |
3 (< 1%) |
| Paresthesia |
9 (2%) |
2 (< 1%) |
6.2 Postmarketing Experience
Cardiovascular:[see Warnings and Precautions (5.2)].
General:
Hepatobiliary:
Local Reactions:
Lower Respiratory:
Neurological:
Skin:
Eye Disorders:
7 DRUG INTERACTIONS
7.1 Drugs Affecting Cytochrome P-450 Enzymes
[see Clinical Pharmacology (12.3)].
7.2 Apomorphine
[see Contraindications (4)].
7.3 Phenytoin, Carbamazepine, and Rifampin
[see Clinical Pharmacology (12.3)].
7.4 Tramadol
7.5 Chemotherapy
7.6 Temazepam
7.7 Alfentanil and Atracurium
Ondansetron does not alter the respiratory depressant effects produced by alfentanil or the degree of neuromuscular blockade produced by atracurium. Interactions with general or local anesthetics have not been studied.
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
[See Clinical Studies (14.2)]. [See Clinical Studies (14.1) and Dosage and Administration (2).]
[See Clinical Pharmacology (12.3).]
8.5 Geriatric Use
[see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
[see Clinical Pharmacology (12.3)].[see Dosage and Administration (2.3)].
8.7 Renal Impairment
[see Clinical Pharmacology (12.3)].
9 DRUG ABUSE AND DEPENDENCE
10 OVERDOSAGE
11 DESCRIPTION
3
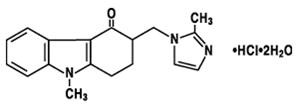
181932
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
3
12.2 Pharmacodynamics
12.3 Pharmacokinetics
| Age-group (years) |
n | Peak Plasma Concentration (ng/mL) |
Mean Elimination Half-life (h) |
Plasma Clearance (L/h/kg) |
|---|---|---|---|---|
| 19 to 40 |
11 |
102 |
3.5 |
0.381 |
| 61 to 74 |
12 |
106 |
4.7 |
0.319 |
| ≥ 75 |
11 |
170 |
5.5 |
0.262 |
Absorption:
Distribution:in vitro
Metabolism:
In vitro in vivoin vivo
in vivo
Elimination:
Geriatrics:
Pediatrics:
| Subjects and Age Group | N | CL (L/h/kg) |
Vdss
(L/kg) |
T½
(h) |
|---|---|---|---|---|
|
a Population PK (Pharmacokinetic) Patients: 64% cancer patients and 36% surgery patients. |
||||
|
Geometric Mean
|
Mean
|
|||
| Pediatric Cancer Patients 4 to 18 years of age |
N = 21 |
0.599 |
1.9 |
2.8 |
| Population PK Patientsa
1 month to 48 months of age |
N = 115 |
0.582 |
3.65 |
4.9 |
,
| Subjects and Age Group | N | CL (L/h/kg) |
Vdss (L/kg) |
T½ (h) |
|---|---|---|---|---|
| Geometric Mean | Mean | |||
| Pediatric Surgery Patients 3 to 12 years of age |
N = 21 |
0.439 |
1.65 |
2.9 |
| Pediatric Surgery Patients 5 to 24 months of age |
N = 22 |
0.581 |
2.3 |
2.9 |
| Pediatric Surgery Patients 1 month to 4 months of age |
N = 19 |
0.401 |
3.5 |
6.7 |
Renal Impairment:
Hepatic Impairment:
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chemotherapy-Induced Nausea and Vomiting
Adults:
Cisplatin-Based Chemotherapy:
| Ondansetron Injection (0.15 mg/kg x 3) |
Placebo | P Valueb | |
|---|---|---|---|
|
a Chemotherapy was high dose (100 and 120 mg/m2; ondansetron injection n = 6, placebo n = 5) or moderate dose (50 and 80 mg/m2; ondansetron injection n = 8, placebo n = 9). Other chemotherapeutic agents included fluorouracil, doxorubicin, and cyclophosphamide. There was no difference between treatments in the types of chemotherapy that would account for differences in response. b Efficacy based on "all patients treated" analysis. c Median undefined since at least 50% of the patients were rescued or had more than five emetic episodes. d Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be. e Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied. |
|||
| Number of patients |
14 |
14 |
|
| Treatment response |
|
|
|
| 0 Emetic episodes 1 to 2 Emetic episodes 3 to 5 Emetic episodes |
2 (14%) 8 (57%) 2 (14%) |
0 (0%) 0 (0%) 1 (7%) |
|
| More than 5 emetic episodes/rescued |
2 (14%) |
13 (93%) |
0.001 |
| Median number of emetic episodes |
1.5 |
Undefinedc
|
|
| Median time to first emetic episode (h) |
11.6 |
2.8 |
0.001 |
| Median nausea scores (0 to 100)d
|
3 |
59 |
0.034 |
| Global satisfaction with control of nausea and vomiting (0 to 100)e
|
96 |
10.5 |
0.009 |
| Ondansetron Injection | Metoclopramide | P Value | |
|---|---|---|---|
|
a In addition to cisplatin, 68% of patients received other chemotherapeutic agents, including cyclophosphamide, etoposide, and fluorouracil. There was no difference between treatments in the types of chemotherapy that would account for differences in response. b Visual analog scale assessment: 0 = not at all satisfied, 100 = totally satisfied. |
|||
| Dose |
0.15 mg/kg x 3 |
2 mg/kg x 6 |
|
| Number of patients in efficacy population |
136 |
138 |
|
| Treatment response |
|
|
|
| 0 Emetic episodes 1 to 2 Emetic episodes 3 to 5 Emetic episodes More than 5 emetic episodes/rescued |
54 (40%) 34 (25%) 19 (14%) 29 (21%) |
41 (30%) 30 (22%) 18 (13%) 49 (36%) |
|
| Comparison of treatments with respect to |
|
|
|
| 0 Emetic episodes More than 5 emetic episodes/rescued |
54/136 29/136 |
41/138 49/138 |
0.083 0.009 |
| Median number of emetic episodes |
1 |
2 |
0.005 |
| Median time to first emetic episode (h) |
20.5 |
4.3 |
< 0.001 |
| Global satisfaction with control of nausea and vomiting (0 to 100)b
|
85 |
63 |
0.001 |
| Acute dystonic reactions |
0 |
8 |
0.005 |
| Akathisia |
0 |
10 |
0.002 |
2
| Ondansetron Injection (0.15 mg/kg x 3) |
Placebo | P Valueb | |
|---|---|---|---|
|
a Chemotherapy consisted of cyclophosphamide in all patients, plus other agents, including fluorouracil, doxorubicin, methotrexate, and vincristine. There was no difference between treatments in the type of chemotherapy that would account for differences in response. b Efficacy based on "all patients treated" analysis. c Median undefined since at least 50% of patients did not have any emetic episodes. d Visual analog scale assessment of nausea: 0 = no nausea, 100 = nausea as bad as it can be. e Visual analog scale assessment of satisfaction: 0 = not at all satisfied, 100 = totally satisfied. |
|||
| Number of patients |
10 |
10 |
|
| Treatment response |
|
|
|
| 0 Emetic episodes |
7 (70%) |
0 (0%) |
0.001 |
| 1 to 2 Emetic episodes 3 to 5 Emetic episodes |
0 (0%) 2 (20%) |
2 (20%) 4 (40%) |
|
| More than 5 emetic episodes/rescued |
1 (10%) |
4 (40%) |
0.131 |
| Median number of emetic episodes |
0 |
4 |
0.008 |
| Median time to first emetic episode (h) |
Undefinedc
|
8.79 |
|
| Median nausea scores (0 to 100)d
|
0 |
60 |
0.001 |
| Global satisfaction with control of nausea and vomiting (0 to 100)e
|
100 |
52 |
0.008 |
Pediatrics:
14.2 Prevention of Postoperative Nausea and/or Vomiting
Adults:
| Ondansetron 4 mg Intravenous |
Placebo | P Value | |
|---|---|---|---|
| Study 1
|
|||
| Emetic episodes: |
|||
| Number of patients |
136
|
139
|
|
| Treatment response over 24-h postoperative period |
|
|
|
| 0 Emetic episodes |
103 (76%) |
64 (46%) |
< 0.001
|
| 1 Emetic episode More than 1 emetic episode/rescued |
13 (10%) 20 (15%) |
17 (12%) 58 (42%) |
|
| Nausea assessments: |
|
|
|
| Number of patients No nausea over 24-h postoperative period |
134 56 (42%) |
136 39 (29%) |
|
| Study 2
|
|||
| Emetic episodes: |
|||
| Number of patients |
136
|
143
|
|
| Treatment response over 24-h postoperative period |
|
|
|
| 0 Emetic episodes |
85 (63%) |
63 (44%) |
0.002
|
| 1 Emetic episode More than 1 emetic episode/rescued |
16 (12%) 35 (26%) |
29 (20%) 51 (36%) |
|
| Nausea assessments: |
|
|
|
| Number of patients No nausea over 24-h postoperative period |
125 48 (38%) |
133 42 (32%) |
P
PP
Pediatrics:
| Treatment Response Over 24 Hours | Ondansetron n (%) |
Placebo n (%) |
P Value |
|---|---|---|---|
|
a Failure was one or more emetic episodes, rescued, or withdrawn. b Nausea measured as none, mild, or severe. |
|||
| Study 1
|
|||
| Number of patients |
205
|
210
|
|
| 0 Emetic episodes |
140 (68%) |
82 (39%) |
≤ 0.001
|
| Failurea
|
65 (32%) |
128 (61%) |
|
| Study 2 |
|
|
|
| Number of patients |
112 |
110 |
|
| 0 Emetic episodes |
68 (61%) |
38 (35%) |
≤ 0.001 |
| Failurea
|
44 (39%) |
72 (65%) |
|
| Study 3 |
|
|
|
| Number of patients |
206 |
206 |
|
| 0 Emetic episodes |
123 (60%) |
96 (47%) |
≤ 0.01 |
| Failurea
|
83 (40%) |
110 (53%) |
|
| Nausea assessmentsb: |
|
|
|
| Number of patients |
185 |
191 |
|
| None |
119 (64%) |
99 (52%) |
≤ 0.01 |
14.3 Prevention of Further Postoperative Nausea and Vomiting
Adults:
| Ondansetron 4 mg Intravenous |
Placebo | P Value | |
|---|---|---|---|
|
a After administration of study drug. b Nausea measured on a scale of 0 to 10 with 0 = no nausea, 10 = nausea as bad as it can be. |
|||
| Study 1 |
|
|
|
| Emetic episodes: |
|
|
|
| Number of patients |
104 |
117 |
|
| Treatment response 24 h after study drug |
|
|
|
| 0 Emetic episodes |
49 (47%) |
19 (16%) |
< 0.001 |
| 1 Emetic episode More than 1 emetic episode/rescued Median time to first emetic episode (min)a |
12 (12%) 43 (41%) 55 |
9 (8%) 89 (76%) 43 |
|
| Nausea assessments: |
|
|
|
| Number of patients Mean nausea score over 24-h postoperative periodb |
98 1.7 |
102 3.1 |
|
| Study 2 |
|
|
|
| Emetic episodes: |
|
|
|
| Number of patients |
112 |
108 |
|
| Treatment response 24 h after study drug |
|
|
|
| 0 Emetic episodes |
49 (44%) |
28 (26%) |
0.006 |
| 1 Emetic episode More than 1 emetic episode/rescued Median time to first emetic episode (min)a |
14 (13%) 49 (44%) 60.5 |
3 (3%) 77 (71%) 34 |
|
| Nausea assessments: |
|
|
|
| Number of patients Mean nausea score over 24-h postoperative periodb |
105 1.9 |
85 2.9 |
|
Repeat Dosing in Adults:
Pediatrics:
|
a Failure was one or more emetic episodes, rescued, or withdrawn. |
|||||||||||||||
|
Treatment Response Over
24 Hours |
Ondansetron
n (%) |
Placebo
n (%) |
P
Value
|
||||||||||||
| Number of patients |
180 |
171 |
|
||||||||||||
| 0 Emetic episodes |
96 (53%) |
29 (17%) |
≤ 0.001 |
||||||||||||
| Failurea
|
84 (47%) |
142 (83%) |
|
||||||||||||
16 HOW SUPPLIED/STORAGE AND HANDLING
Retain in carton until time of use.
17 PATIENT COUNSELING INFORMATION
- Patients should be informed that ondansetron may cause serious cardiac arrhythmias such as QT prolongation. Patients should be instructed to tell their health care provider right away if they perceive a change in their heart rate, if they feel lightheaded, or if they have a syncopal episode.
- Patients should be informed that the chances of developing severe cardiac arrhythmias such as QT prolongation and Torsade de Pointes are higher in the following people:
- Patients with a personal or family history of abnormal heart rhythms, such as congenital long QT syndrome;
- Patients who take medications, such as diuretics, which may cause electrolyte abnormalities
- Patients with hypokalemia or hypomagnesemia
Ondansetron should be avoided in these patients, since they may be more at risk for cardiac arrhythmias such as QT prolongation and Torsade de Pointes.
- Inform patients that ondansetron may cause hypersensitivity reactions, some as severe as anaphylaxis and bronchospasm. The patient should report any signs and symptoms of hypersensitivity reactions, including fever, chills, rash, or breathing problems.
- The patient should report the use of all medications, especially apomorphine, to their health care provider. Concomitant use of apomorphine and ondansetron may cause a significant drop in blood pressure and loss of consciousness.
- Inform patients that ondansetron may cause headache, drowsiness/sedation, constipation, fever and diarrhea.
Manufactured for:
AuroMedics Pharma LLC
6 Wheeling Road
Dayton, NJ 08810
Manufactured by:
Aurobindo Pharma Limited
IDA, Pashamylaram - 502307
AP., India
Revised: December 2012
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg/2 mL Container Label
NDC 55150-125-02
ONDANSETRON
INJECTION, USP
4 mg/2 mL (2 mg/mL)
For I.V. or I.M. Injection.
Rx only 2 mL Single-dose Vial
AUROMEDICS

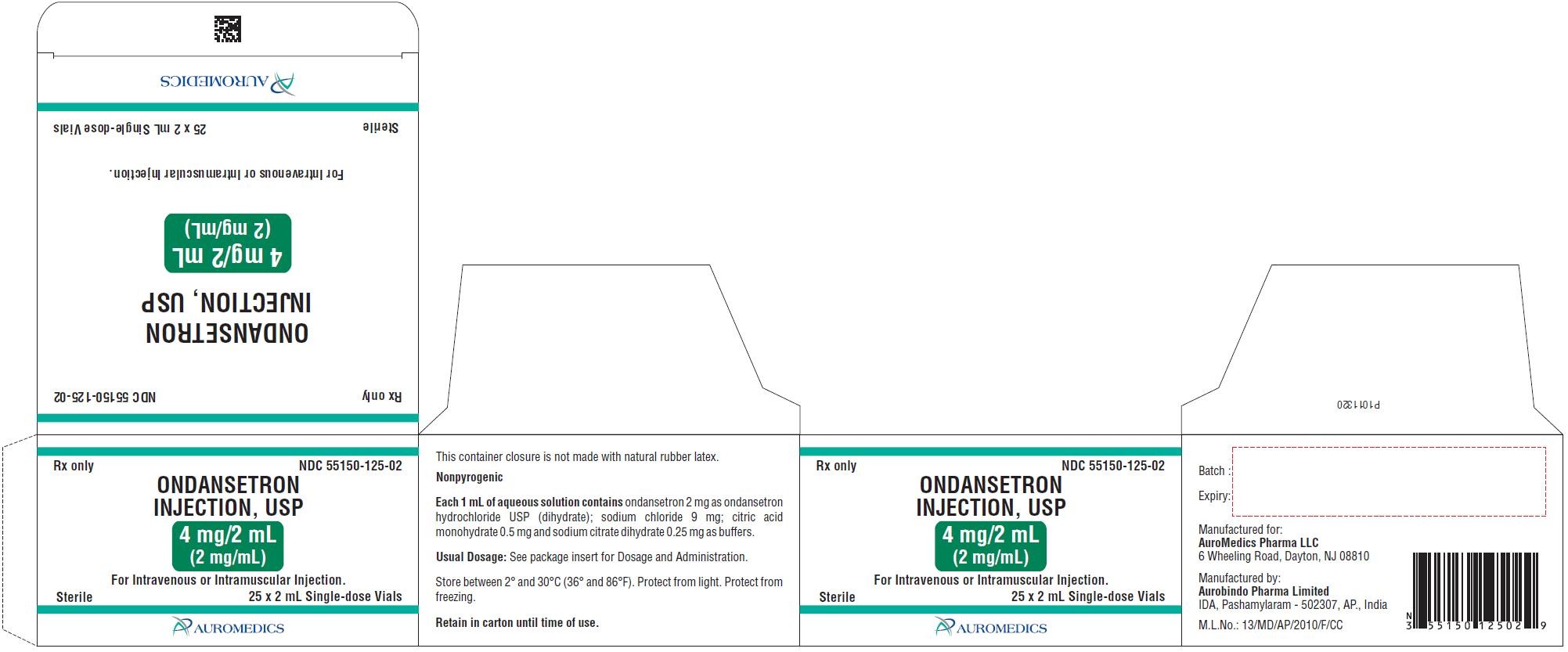
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 4 mg/2 mL Container-Carton (25 Vials)
Rx onlyNDC 55150-125-02
ONDANSETRON
INJECTION, USP
4 mg/2 mL
(2 mg/mL)
For Intravenous or Intramuscular Injection.
Sterile 25 x 2 mL Single-dose Vials
AUROMEDICS
OndansetronOndansetron Hydrochloride INJECTION, SOLUTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||