Nystatin
Nystatin Cream USP, 100,000 units per gram
FULL PRESCRIBING INFORMATION: CONTENTS*
- NYSTATIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- NYSTATIN INDICATIONS AND USAGE
- NYSTATIN CONTRAINDICATIONS
- PRECAUTIONS
- NYSTATIN ADVERSE REACTIONS
- NYSTATIN DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Rx only
NYSTATIN DESCRIPTION
Nystatin Cream is for dermatologic use.
Nystatin is a polyene antimycotic obtained from Streptomyces noursei. It is a yellow to light tan powder with a cereal-like odor, very soluble in water, and slightly to sparingly soluble in alcohol. Structural formula:
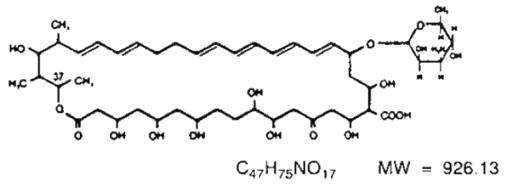
Nystatin Cream contains the antifungal antibiotic nystatin at a concentration of 100,000 USP Nystatin Units per gram in an aqueous, perfumed cream base containing purified water, propylene glycol, methylparaben, propylparaben, white petrolatum, glyceryl monostearate, polyethylene glycol 400 monostearate, ceteareth-15, medical antifoam AF emulsion, aluminum hydroxide gel, titanium dioxide, sorbitol solution, and, if necessary, sodium hydroxide for pH adjustment.
CLINICAL PHARMACOLOGY
Nystatin is an antifungal antibiotic which is both fungistatic and fungicidal in vitro against a wide variety of yeasts and yeast-like fungi. It probably acts by binding to sterols in the cell membrane of the fungus with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin is the first well tolerated antifungal antibiotic of dependable efficacy for the treatment of cutaneous, oral and intestinal infections caused by Candida (Monilia) albicans and other Candida species. It exhibits no appreciable activity against bacteria.
Nystatin provides specific therapy for all localized forms of candidiasis. Symptomatic relief is rapid, often occurring within 24 to 72 hours after the initiation of treatment. Cure is effected both clinically and mycologically in most cases of localized candidiasis.
NYSTATIN INDICATIONS AND USAGE
Nystatin Cream is indicated in the treatment of cutaneous or mucocutaneous mycotic infections caused by Candida (Monilia) albicans and other Candida species.
NYSTATIN CONTRAINDICATIONS
Nystatin Cream is contraindicated in patients with a history of hypersensitivity to any of its components.
PRECAUTIONS
Should a reaction of hypersensitivity occur the drug should be immediately withdrawn and appropriate measures taken.
Nystatin Cream is not for ophthalmic use.
NYSTATIN ADVERSE REACTIONS
Nystatin is virtually nontoxic and nonsensitizing and is well tolerated by all age groups including debilitated infants, even on prolonged administration. If irritation on topical application should occur, discontinue medication.
NYSTATIN DOSAGE AND ADMINISTRATION
Nystatin Cream should be applied liberally to affected areas twice daily or as indicated until healing is complete.
Nystatin Cream is usually preferred to Nystatin Ointment in candidiasis involving intertriginous areas; very moist lesions, however, are best treated with Nystatin Topical Powder.
HOW SUPPLIED
Nystatin Cream is a smooth yellow cream with a characteristic perfume odor.
Nystatin Cream is supplied in 15g (NDC 21695-761-15) and 30g (NDC 21695-761-30) tubes providing 100,000 USP Nystatin Units per gram.
Store at 20°-25°C (68°-77°F) [see USP Controlled Room Temperature].
Mfd. by: Taro Pharmaceuticals Inc.
Brampton, Ontario, Canada L6T 1C1
Dist. by: Taro Pharmaceuticals U.S.A., Inc.
Hawthorne, NY 10532
Repackaged by:
Rebel Distributors Corp
Thousand Oaks, CA 91320
Principal Display Panel

NystatinNystatin CREAM
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||