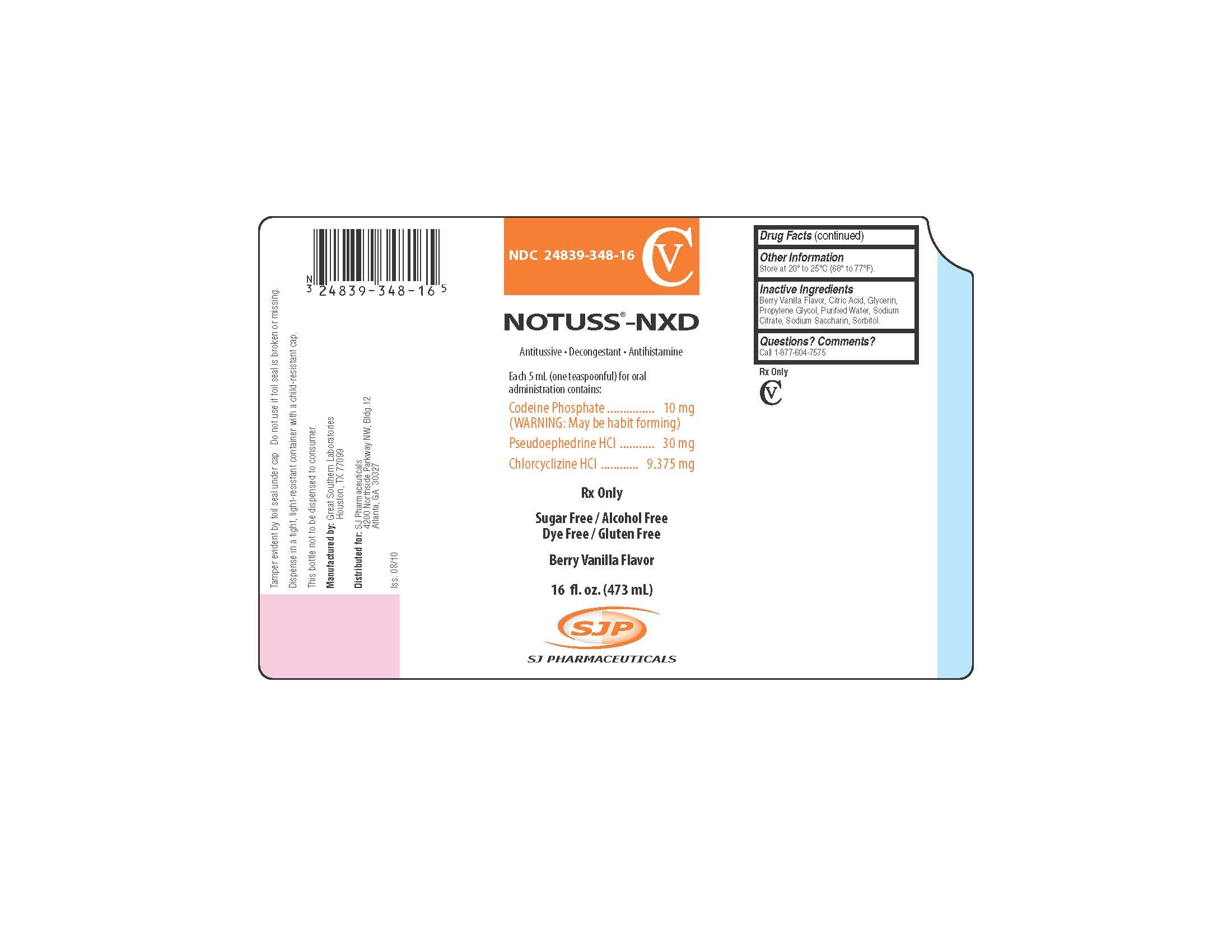
Notuss-NXD
SJ PHARMACEUTICALS, LLC
Great Southern Laboratories
Notuss-NXD
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
DESCRIPTION:
Each 5 mL (one teaspoonful) for oral administration contains:
Codeine Phosphate .................................................. 10 mg
(WARNING: May be habit forming)
Pseudoephedrine HCl .............................................. 30 mg
Chlorcyclizine HCl ............................................... 9.375 mg
Active Ingredients Purpose
(in each 5 mL teaspoonful)
Codeine Phosphate 10 mg .................................. Antitussive
Pseudoephedrine Hydrochloride 30 mg ........... Decongestant
Chlorcyclizine Hydrochloride 9.375 mg .............. Antihistamine
USES:
WARNINGS:
Do not exceed recommended dosage.
Do not use this product
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to the enlargement of the prostate gland
- a cough that lasts or is chronic such as occurs with smoking, asthma or emphysema
- a cough that occurs with too much phlegm
- a chronic pulmonary disease or shortness of breath, or children who are taking other drugs
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- excitability may occur, especially in children
- may cause marked drowsiness
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- may cause or aggravate constipation
- nervousness, dizziness, or sleeplessness occurs
- cough or nasal congestion persists for more than 1 week, tends to recur, or is accompanied by a fever, rash or persistent headache. These could be signs of a serious condition.
- new symptoms occur
DIRECTIONS:
PRODUCT PACKAGING:
NOTUSS®-NXD
Antitussive - Decongestant - Antihistamine
Sugar Free/Alcohol Free
Dye Free/Gluten Free
16 fl. oz. (473 mL)
Tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
Dispense in a tight, light-resistant container with a child-resistant cap.
This bottle not to be dispensed to consumer.
Manufactured by:
Great Southern Laboratories
Houston, TX 77099

Notuss-NXDCodeine Phosphate, Pseudoephedrine HCl, Chlorcyclizine HCl LIQUID
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||