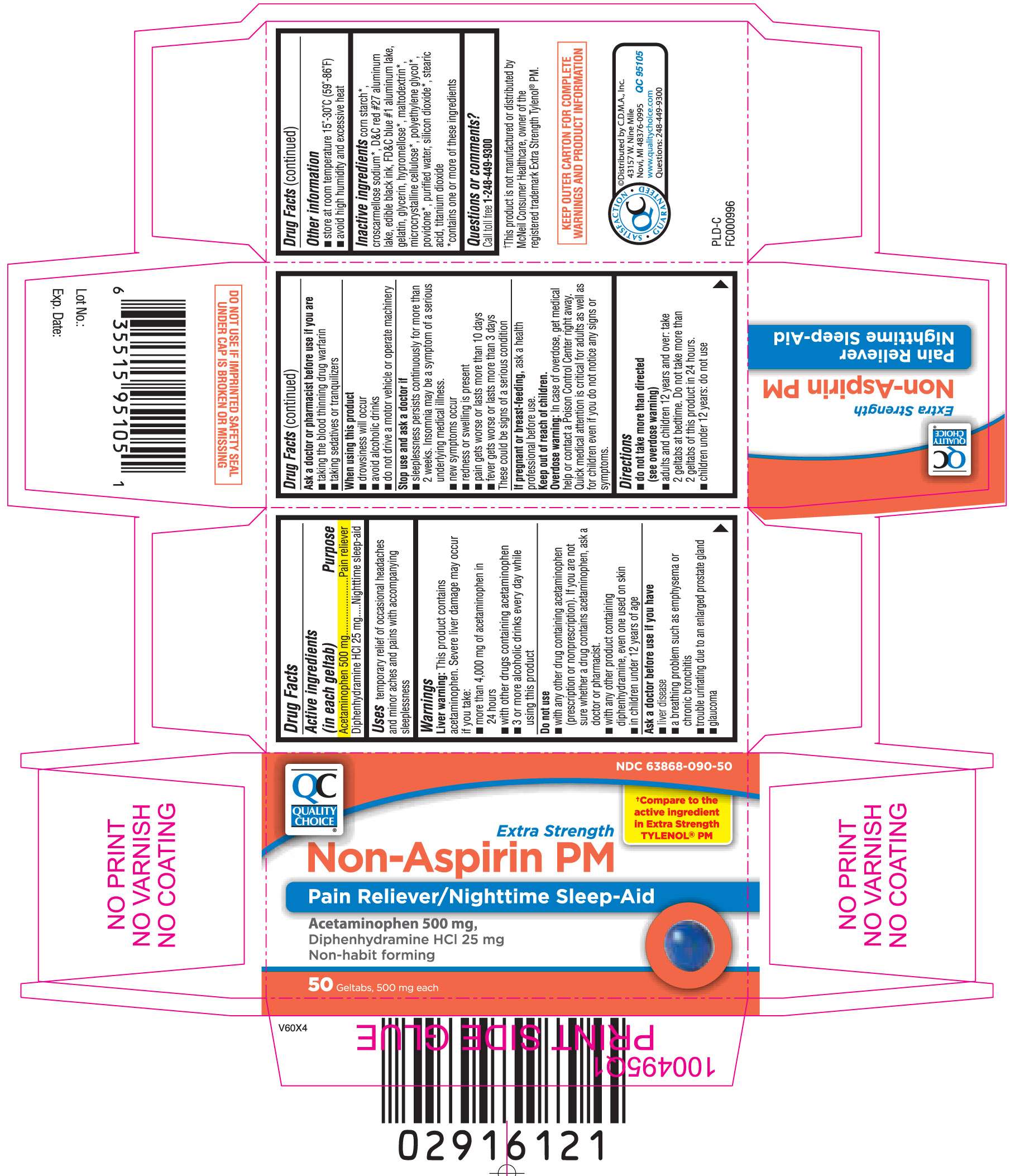
Non Aspirin PM
QUALITY CHOICE (Chain Drug Marketing Association)
P and L Development of New York Corporation
DRUG FACTS
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredients (in each geltab)
- Purpose
- Non Aspirin PM Uses
- Warnings
- Directions
- Non Aspirin PM Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
- Product Label
FULL PRESCRIBING INFORMATION
Active ingredients (in each geltab)
Acetaminophen 500 mg
Diphenhydramine HCl 25 mg
Purpose
Pain reliever
Nighttime sleep-aid
Non Aspirin PM Uses
temporary relief of occasional headaches and minor aches and pains with accompanying sleeplessness
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other product containing diphenhydramine, even one used on skin
- in children under 12 years of age
Ask a doctor before use if you have
- liver disease
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
- glaucoma
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers
When using this product
- drowsiness will occur
- avoid alcoholic drinks
- do not drive a motor vehicle or operate machinery
Stop use and ask a doctor if
- sleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
- new symptoms occur
- redness or swelling is present
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
These could be signs of a serious condition.
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
Overdose warning: In case of overdose, get medical help or contact a Poison Control Center right away. Quick medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years and over: take 2 geltabs at bedtime. Do not take more than 2 geltabs of this product in 24 hours.
- children under 12 years: do not use
Non Aspirin PM Other information
- store at room temperature 15º-30ºC (59º-86ºF)
- avoid high humidity and excessive heat
Inactive ingredients
corn starch*, croscarmellose sodium*, D&C red #27 aluminum lake, edible black ink, FD&C blue #1 aluminum lake, gelatin, glycerin, hypromellose*, maltodextrin*, microcrystalline cellulose*, polyethylene glycol*, povidone*, purified water, silicon dioxide*, stearic acid, titanium dioxide
*contains one or more of these ingredients
Questions or comments?
Call toll free 1-248-449-9300
Principal Display Panel
†Compare to active ingredient in Extra Strength TYLENOL® PM
Extra Strength Non-Aspirin PM
Pain Reliever/Nighttime Sleep-Aid
Acetaminophen 500 mg,
Diphenhydramine HCl 25 mg
Non-habit forming
Geltabs, 500 mg each
†This product is not manufactured or distributed by McNeil Consumer Healthcare, owner of the registered trademark Extra Strength Tylenol® PM.
KEEP OUTER CARTON FOR COMPLETE WARNINGS AND PRODUCT INFORMATION
©Distributed by C.D.M.A., Inc.
43157 W. Nine Mile
Novi, MI 48376-0995
Questions: 248-449-9300
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Product Label
Non Aspirin PMAcetaminophen, Diphenhydramine HCl TABLET, COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||