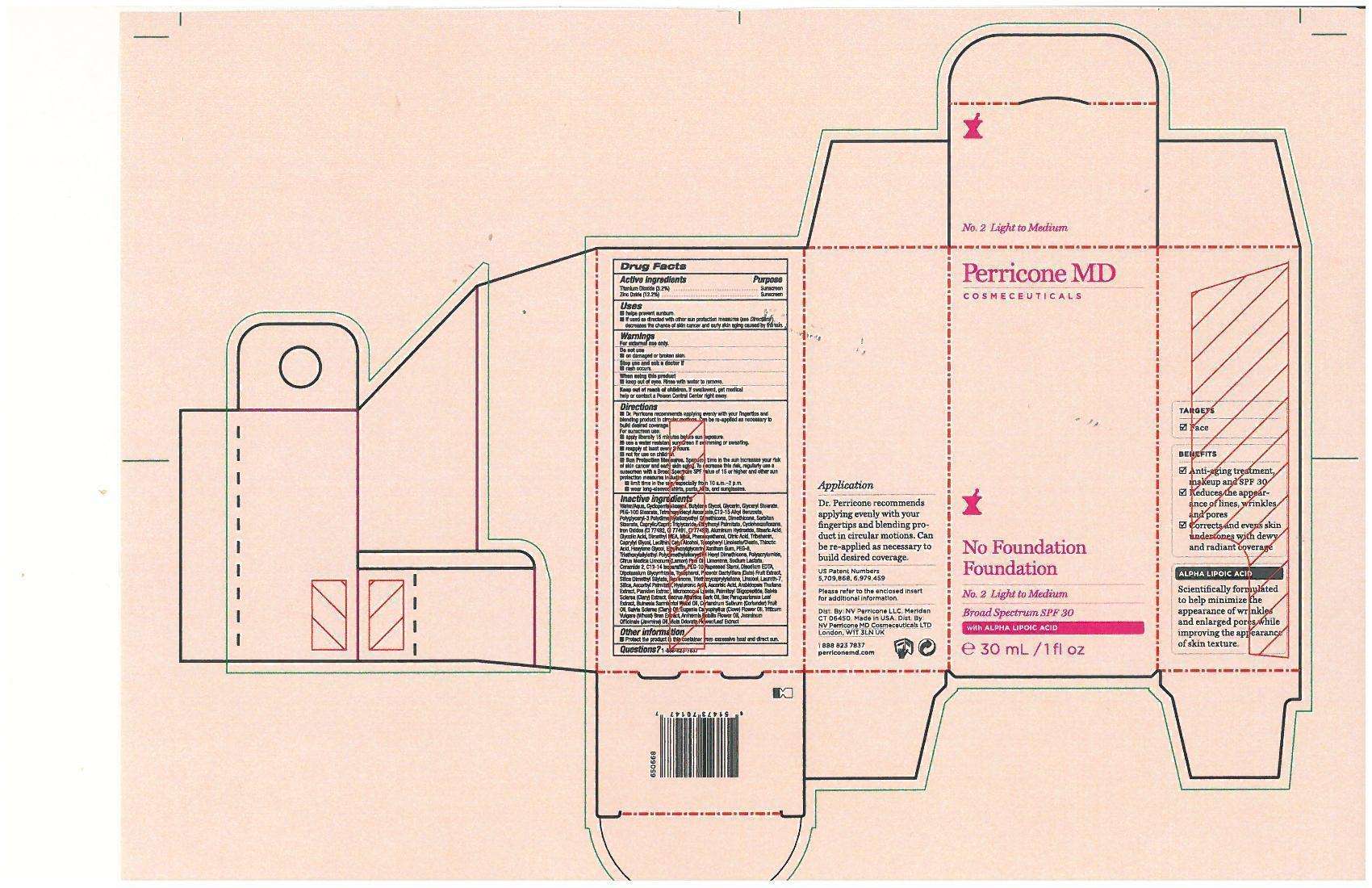
No Foundation Foundation No.2 Light to Medium
Allure Labs, Inc.
Allure Labs, Inc.
Drug Facts
FULL PRESCRIBING INFORMATION
Purpose
PURPOSE:
Sunscreen
Uses
USES:
Helps prevent sunburn
If used as directed with other sun protection measures (see directions) decreases the chance of skin cancer and early skin aging caused by the sun.
WARNINGS:
- For external use only
WHEN USING THIS PRODUCT:
- Keep out of eyes. Rinse with water to remove.
STOP USE AND ASK A DOCTOR IF
- Rash occurs.
KEEP OUT OF REACH OF CHILDREN
If swallowed, get medical help or contact a Poison Control Center right away.
Uses
DIRECTIONS:
- Dr. Perricone recommends applying evenly with your fingertips and blending product in circular motions. Can be re-applied as necessary to build desired coverage.
- use liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least evry 2 hours
- Not for use on children.
- Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease the risk, regularly use a sunscreen with a Broad Spectrun SPF value of 15 or higher and other sun protection measures including:
- Limit time in the sun, especially from 10:00am -2pm.
- wear long-sleeved shirts, pants, hats and sunglasses.
Other Information:
- Protect the production this container from excessive heat and direct sun.
INACTIVE INGREDIENTS:
Aqua (Water), Cyclopentasiloxane, Butylene Glycol, Glycerin, Glyceryl Stearate, PEG-100 Stearate, Tetrahexyldecyl Ascorbate, C12-15 Alkyl Benzoate, Polyglyceryl-3 Polydimethylsiloxyethyl Dimethicone,Dimethicone, Sorbitan Stearate, Caprylic/Capric Triglyceride, Ethylhexyl Palmitate, Cyclohexasiloxane, Iron Oxides (CI77492, CI77491, CI77499), Aluminum Hydroxide, Stearic AcidGlycolic Acid, Dimethyl MEA (DMAE), Mica, Phenoxyethanol, Citric Acid, Tribehenin, Caprylyl Glycol, Lecithin, Cetyl Alcohol, Tocopheryl Linoleate/Oleate, Thioctic Acid (Alpha-Lipoic Acid), Hexylene Glycol, Ethylhexylglycerin, Xanthan Gum, PEG-8, Triethoxysilylethyl Polydimethylsilxyethyl Hexyl Dimethicone, Polyacrylamide, Citrus Medica Limonum (Lemon) Peel Oil, Limonene, Sodium Lactate, Ceramide-2, Aluminum Hydroxide,C13-14 Isoparaffin, PEG-10 Rapeseed Sterol, Disodium EDTA, Dipotassium Glycyrrhizate, Tocopherol, Phoenix Dactylifera (Date) Fruit Extract, Silica Dimethyl Silylate, Teprenone, Triethoxycaprylylsilane, Linalool, Laureth-7, Silica, Ascorbyl Palmitate, Hyaluronic Acid, Ascorbic Acid, Arabidopsis Thaliana Extract, Plankton Extract, Micrococcus Lysate, Palmitoyl Oligopeptide, Salvia Sclarea (Clary) Extract, Cedrus Atlantica Bark Oil, Ilex Paraguariensis Leaf Extract, Bulnesia SarmientoiWood Oil, Coriandrm Sativum (Coriander) Fruit Oil, Salvia Sclarea (Clary) Oil, Eugenia Caryophyllus (Clove) Flower Oil, Triticum Vulgare (Wheat) Bran Extract, Anthemis Nobilis Flower Oil, Jasminum Officinale (Jasmine) Oil, Viola Odorata Flower/Leaf Extract.
DISTRIBUTOR (US):
NV Perricone LLC,
Meriden CT 06450.
DISTRIBUTOR (UK):
NV Perricone MD Cosmeceuticals LTD
London, W1T 3LN UK
www.perriconemd.com
1 888 823 7837
IMAGE OF THE PRODUCT: