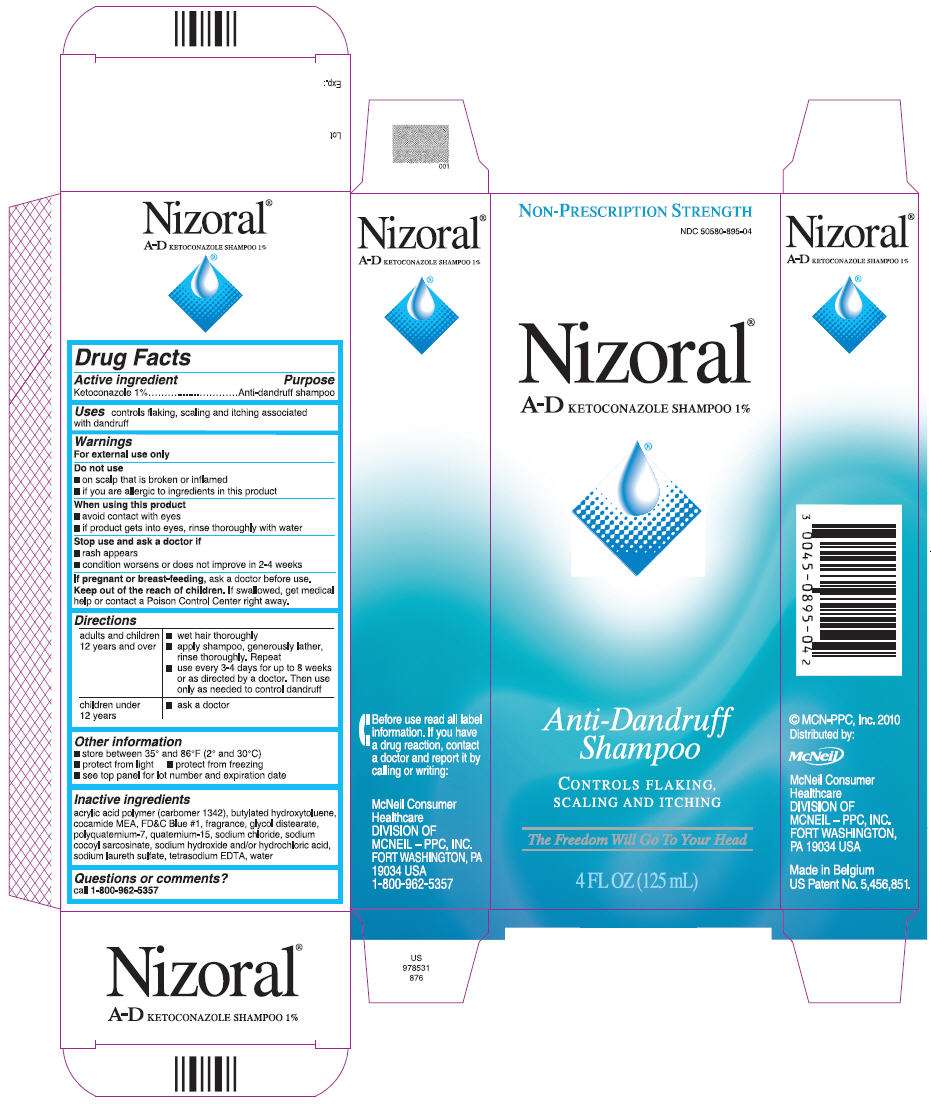
Nizoral
McNeil Consumer Healthcare Div. McNeil-PPC, Inc
Nizoral A-D KETOCONAZOLE SHAMPOO 1%
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient
- Purpose
- Nizoral Uses
- Warnings
- Directions
- Nizoral Other information
- Inactive ingredients
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
Drug Facts
Active ingredient
Ketoconazole 1%
Purpose
Anti-dandruff shampoo
Nizoral Uses
controls flaking, scaling and itching associated with dandruff
Warnings
For external use only
Do not use
- on scalp that is broken or inflamed
- if you are allergic to ingredients in this product
When using this product
- avoid contact with eyes
- if product gets into eyes, rinse thoroughly with water
Stop use and ask a doctor if
- rash appears
- condition worsens or does not improve in 2-4 weeks
If pregnant or breast-feeding, ask a doctor before use
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
Directions
| adults and children 12 years and over |
|
| children under 12 years |
|
Nizoral Other information
- store between 35° and 86°F (2° and 30°C)
- protect from light
- protect from freezing
- see top panel for lot number and expiration date
Inactive ingredients
acrylic acid polymer (carbomer 1342), butylated hydroxytoluene, cocamide MEA, FD&C Blue #1, fragrance, glycol distearate, polyquaternium-7, quaternium-15, sodium chloride, sodium cocoyl sarcosinate, sodium hydroxide and/or hydrochloric acid, sodium laureth sulfate, tetrasodium EDTA, water-
Questions or comments?
call 1-800-962-5357
PRINCIPAL DISPLAY PANEL
NON-PRESCRIPTION STRENGTH
NDC 50580-895-04
Nizoral®
A-D KETOCONAZOLE SHAMPOO 1%
Anti-Dandruff
Shampoo
CONTROLS FLAKING,
SCALING AND ITCHING
The Freedom Will Go To Your Head
4 FL OZ (125 mL)
NizoralKetoconazole SHAMPOO
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||