Nizatidine
Bryant Ranch Prepack
Bryant Ranch Prepack
NIZATIDINE CAPSULES
FULL PRESCRIBING INFORMATION: CONTENTS*
- NIZATIDINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- NIZATIDINE INDICATIONS AND USAGE
- CONTRAINDICATION
- PRECAUTIONS
- NIZATIDINE ADVERSE REACTIONS
- OVERDOSAGE
- NIZATIDINE DOSAGE AND ADMINISTRATION
- Nizatidine 300mg Capsule
FULL PRESCRIBING INFORMATION
NIZATIDINE DESCRIPTION
Nizatidine, USP is a histamine H -receptor antagonist. Chemically, it is N-[2-[[[2-[(dimethylamino)methyl]-4-thiazolyl]methyl]thio]ethyl]-N'-methyl-2-nitro-1,1-ethenediamine. 2
The structural formula is as follows:
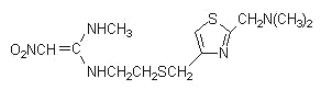
Nizatidine has the empirical formula C H N O S representing a molecular weight of 331.46. It is an off white to buff crystalline solid that is soluble in water. Nizatidine has a bitter taste and mild sulfur-like odor. Each capsule contains for oral administration 150 mg (0.45 mmol) or 300 mg (0.91 mmol) of nizatidine and the following inactive ingredients: croscarmellose sodium, povidone, starch, dimethicone and talc. The capsule shells contain D&C yellow no. 10, Titanium Dioxide, Gelatin, D&C red no. 28, FD&C blue no. 1, FD&C red no. 40 (for 150 mg) and Titanium Dioxide, Gelatin, D&C yellow no.10, FD&C blue no.1, FD&C red no. 40 (for 300 mg). The imprinting ink contains Shellac, Iron Oxide Black, N-Butyl Alcohol, Propylene Glycol, FD&C blue no. 2, FD&C red no. 40, FD&C blue no. 1, D&C yellow no. 10, SDA 3A Alcohol (for 150 mg) and Shellac, Dehydrated Alcohol, Isopropyl Alcohol, Butyl Alcohol, Propylene Glycol, Strong Ammonia Solution, Black Iron Oxide, Potassium Hydroxide (for 300 mg). 12 21 5 2 2
CLINICAL PHARMACOLOGY
Nizatidine is a competitive, reversible inhibitor of histamine at the histamine H -receptors, particularly those in the gastric parietal cells. 2
Clinical Trials
NIZATIDINE INDICATIONS AND USAGE
Nizatidine capsules are indicated for up to 8 weeks for the treatment of active duodenal ulcer. In most patients, the ulcer will heal within 4 weeks.
Nizatidine capsules are indicated for maintenance therapy for duodenal ulcer patients at a reduced dosage of 150 mg h.s. after healing of an active duodenal ulcer. The consequences of continuous therapy with nizatidine for longer than 1 year are not known.
Nizatidine capsules are indicated for up to 12 weeks for the treatment of endoscopically diagnosed esophagitis, including erosive and ulcerative esophagitis, and associated heartburn due to GERD.
Nizatidine capsules are indicated for up to 8 weeks for the treatment of active benign gastric ulcer. Before initiating therapy, care should be taken to exclude the possibility of malignant gastric ulceration.
CONTRAINDICATION
Nizatidine capsules are contraindicated in patients with known hypersensitivity to the drug. Because cross sensitivity in this class of compounds has been observed, H -receptor antagonists, including nizatidine, should not be administered to patients with a history of hypersensitivity to other H -receptor antagonists. 2 2
PRECAUTIONS
General
- Symptomatic response to nizatidine therapy does not preclude the presence of gastric malignancy.
- Because nizatidine is excreted primarily by the kidney, dosage should be reduced in patients with moderate to severe renal insufficiency ( ). see DOSAGE AND ADMINISTRATION
- Pharmacokinetic studies in patients with hepatorenal syndrome have not been done. Part of the dose of nizatidine is metabolized in the liver. In patients with normal renal function and uncomplicated hepatic dysfunction, the disposition of nizatidine is similar to that in normal subjects.
Laboratory Tests
False-positive tests for urobilinogen with Multistix may occur during therapy with nizatidine. ®
Drug Interactions
No interactions have been observed between nizatidine and theophylline, chlordiazepoxide, lorazepam, lidocaine, phenytoin, and warfarin. Nizatidine does not inhibit the cytochrome P-450-linked drug-metabolizing enzyme system; therefore, drug interactions mediated by inhibition of hepatic metabolism are not expected to occur. In patients given very high doses (3,900 mg) of aspirin daily, increases in serum salicylate levels were seen when nizatidine, 150 mg b.i.d., was administered concurrently.
Carcinogenesis, Mutagenesis, Impairment of Fertility
A 2-year oral carcinogenicity study in rats with doses as high as 500 mg/kg/day (about 80 times the recommended daily therapeutic dose) showed no evidence of a carcinogenic effect. There was a dose-related increase in the density of enterochromaffin-like (ECL) cells in the gastric oxyntic mucosa. In a 2-year study in mice, there was no evidence of a carcinogenic effect in male mice; although hyperplastic nodules of the liver were increased in the high-dose males as compared with placebo. Female mice given the high dose of nizatidine (2,000 mg/kg/day, about 330 times the human dose) showed marginally statistically significant increases in hepatic carcinoma and hepatic nodular hyperplasia with no numerical increase seen in any of the other dose groups. The rate of hepatic carcinoma in the high-dose animals was within the historical control limits seen for the strain of mice used. The female mice were given a dose larger than the maximum tolerated dose, as indicated by excessive (30%) weight decrement as compared with concurrent controls and evidence of mild liver injury (transaminase elevations). The occurrence of a marginal finding at high dose only in animals given an excessive and somewhat hepatotoxic dose, with no evidence of a carcinogenic effect in rats, male mice, and female mice (given up to 360 mg/kg/day, about 60 times the human dose), and a negative mutagenicity battery are not considered evidence of a carcinogenic potential for nizatidine.
Nizatidine was not mutagenic in a battery of tests performed to evaluate its potential genetic toxicity, including bacterial mutation tests, unscheduled DNA synthesis, sister chromatid exchange, mouse lymphoma assay, chromosome aberration tests, and a micronucleus test.
In a 2-generation, perinatal and postnatal fertility study in rats, doses of nizatidine up to 650 mg/kg/day produced no adverse effects on the reproductive performance of parental animals or their progeny.
Pregnancy
Teratogenic Effects
Nursing Mothers
Studies conducted in lactating women have shown that 0.1% of the administered oral dose of nizatidine is secreted in human milk in proportion to plasma concentrations. Because of the growth depression in pups reared by lactating rats treated with nizatidine, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Of the 955 patients in clinical studies who were treated with nizatidine, 337 (35.3%) were 65 and older. No overall differences in safety or effectiveness were observed between these and younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function ( ). see DOSAGE AND ADMINISTRATION
NIZATIDINE ADVERSE REACTIONS
Worldwide, controlled clinical trials of nizatidine included over 6,000 patients given nizatidine in studies of varying durations. Placebo-controlled trials in the United States and Canada included over 2,600 patients given nizatidine and over 1,700 given placebo. Among the adverse events in these placebo-controlled trials, anemia (0.2% vs 0%) and urticaria (0.5% vs 0.1%) were significantly more common in the nizatidine group.
OVERDOSAGE
Overdoses of nizatidine have been reported rarely. The following is provided to serve as a guide should such an overdose be encountered.
NIZATIDINE DOSAGE AND ADMINISTRATION
Nizatidine 300mg Capsule

NizatidineNizatidine CAPSULE
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||