NITROGEN
Aspen Air US Corp
Aspen Air US Corp
FULL PRESCRIBING INFORMATION: CONTENTS*
FULL PRESCRIBING INFORMATION
NITROGEN CERTIFICATE OF ANALYSIS
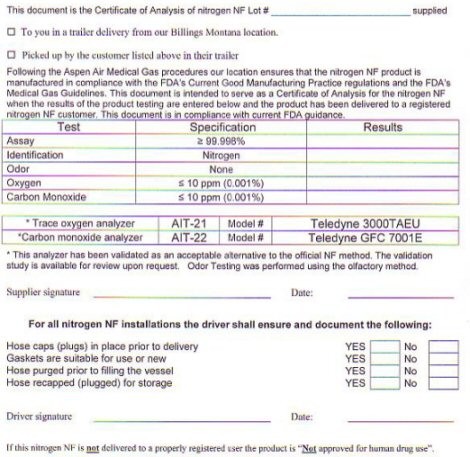
THIS DOCUMENT IS THE CERTIFICATE OF ANALYSIS OF NITROGEN NF LOT # ______________________ SUPPLIED TO YOU IN A TRAILER FROM OUR BILLINGS MONTANA LOCATION OR PICKED UP BY THE CUSTOMER LISTED ABOVE IN THEIR TRAILER. FOLLOWING THE ASPEN AIR MEDICAL GAS PROCEDURES OUR LOCATION ENSURES THAT THE OXYGEN USP PRODUCT IS MANUFACTURED IN COMPLIANCE WITH THE FDA'S CURRENT GOOD MANUFACTURING PRACTICE REGULATIONS AND THE FDA'S MEDICAL GAS GUIDELINES. THIS DOCUMENT IS INTENDED TO SERVE AS A CERTIFICATE OF ANALYSIS FOR THE OXYGEN USP WHEN THE RESULTS OF THE PRODUCT TESTING ARE ENTERED BELOW AND THE PRODUCT HAS BEEN DELIVERED TO A REGISTERED OXYGEN USP CUSTOMER. THIS DOCUMENT IS IN COMPLIANCE WITH CURRENT FDA GUIDANCE
TEST SPECIFICATIONS RESULTS
ASSAY GREATER THAN 99.998%
IDENTIFICATION NITROGEN
ODOR NONE
OXYGEN LESS THAN 0.001%
CARBON MONOXIDE LESS THAN 0.001%
THE METHODOLOGY USED FOR PERFORMING THE USP TEST FOR ASSAY AND IDENTIFICATION IS THE PARAMAGNETIC ANALYZER MODEL # TELEDYNE 2010 MA. THIS ANALYZER HAS BEEN VALIDATED AS AN ACCEPTABLE ALTERNATIVE TO THE OFFICIAL USP METHOD FOR OXYGEN ASSAY AND IDENTIFICATION. THE VALIDATION STUDY IS AVAILABLE FOR REVIEW UPON REQUEST. ODOR TESTING WAS PERFORMED BY THE OLFACTORY METHOD. SUPPLIER SIGNATURE _________________________ DATE _________________
NUMBER 33 110 A1 - REVISION DATE 05/01/08
GENERAL PRECAUTIONS
If this NITROGEN NF is not delivered to a properly registered
user the product is “Not approved for human drug use”.
This product has been produced by the air liquefaction
process and is exempt from these tests as stated in the USP / NF monograph for
NITROGEN
FOR ALL NITROGEN N INSTALLATIONS THE DRIVER SHALL ENSURE AND DOCUMENT THE FOLLOWING: HOSE CAPS (PLUGS) IN PLACE PRIOR TO DELIVERY YES/NO, GASKETS ARE SUITABLE FOR USE OR NEW YES/NO, HOSE PURGED PRIOR TO FILLING THE VESSEL YES/NO, HOSE RE-CAPED (PLUGGED) FOR STORAGE YES/NO. DRIVER SIGNATURE:____________________ DATE:_________________
NITROGENNITROGEN GAS
| |||||||||||||||||||||||||||||||||||||||||||||||||