Nitrofurantoin
FULL PRESCRIBING INFORMATION: CONTENTS*
- NITROFURANTOIN DESCRIPTION
- CLINICAL PHARMACOLOGY
- MICROBIOLOGY
- INDICATIONS & USAGE
- NITROFURANTOIN CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- NITROFURANTOIN ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
NITROFURANTOIN DESCRIPTION
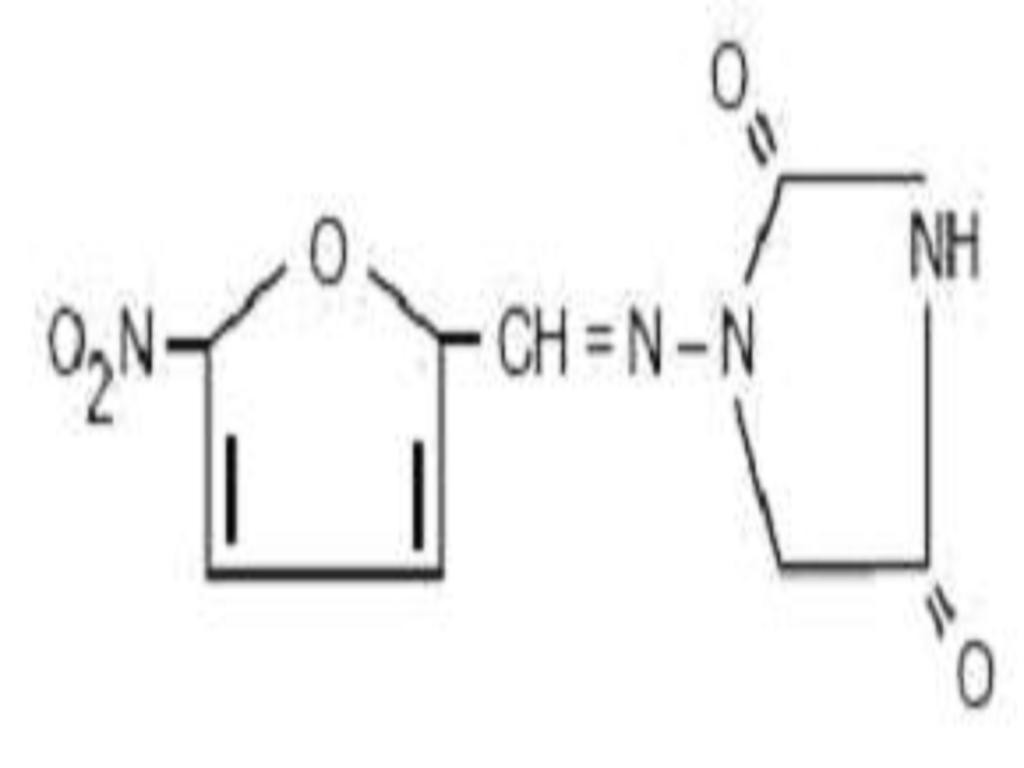
CLINICAL PHARMACOLOGY
MICROBIOLOGY
Gram-Positive Aerobes
Gram-Negative Aerobes
Gram-Positive Aerobes
Susceptibility Tests
Dilution Techniques
Diffusion Techniques
INDICATIONS & USAGE
NITROFURANTOIN CONTRAINDICATIONS
WARNINGS
Pulmonary ReactionsACUTE, SUBACUTE, OR CHRONIC PULMONARY REACTIONS HAVE BEEN OBSERVED IN PATIENTS TREATED WITH NITROFURANTOIN. IF THESE REACTIONS OCCUR, NITROFURANTOIN MACROCRYSTALS SHOULD BE DISCONTINUED AND APPROPRIATE MEASURES TAKEN. REPORTS HAVE CITED PULMONARY REACTIONS AS A CONTRIBUTING CAUSE OF DEATH.
CHRONIC PULMONARY REACTIONS (DIFFUSE INTERSTITIAL PNEUMONITIS OR PULMONARY FIBROSIS, OR BOTH) CAN DEVELOP INSIDIOUSLY. THESE REACTIONS OCCUR RARELY AND GENERALLY IN PATIENTS RECEIVING THERAPY FOR SIX MONTHS OR LONGER. CLOSE MONITORING OF THE PULMONARY CONDITION OF PATIENTS RECEIVING LONG-TERM THERAPY IS WARRANTED AND REQUIRES THAT THE BENEFITS OF THERAPY BE WEIGHED AGAINST POTENTIAL RISKS (SEE RESPIRATORY REACTIONS).
Hepatotoxicity
Neuropathy
Hemolytic Anemia
Clostridium difficile-associated diarrhea
PRECAUTIONS
Information for PatientsGeneral
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy Category B
Non-Teratogenic Effects
Nitrofurantoin has been shown in one published transplacental carcinogenicity study to induce lung papillary adenomas in the F1 generation mice at doses 19 times the human dose on a mg/kg basis. The relationship of this finding to potential human carcinogenesis is presently unknown. Because of the uncertainty regarding the human implications of these animal data, this drug should be used during pregnancy only if clearly needed.
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
GERIATRIC USE
NITROFURANTOIN ADVERSE REACTIONS
Respiratory
CHRONIC, SUBACUTE, OR ACUTE PULMONARY HYPERSENSITIVITY REACTIONS MAY OCCUR.
CHRONIC PULMONARY REACTIONS OCCUR GENERALLY IN PATIENTS WHO HAVE RECEIVED CONTINUOUS TREATMENT FOR SIX MONTHS OR LONGER. MALAISE, DYSPNEA ON EXERTION, COUGH, AND ALTERED PULMONARY FUNCTION ARE COMMON MANIFESTATIONS WHICH CAN OCCUR INSIDIOUSLY. RADIOLOGIC AND HISTOLOGIC FINDINGS OF DIFFUSE INTERSTITIAL PNEUMONITIS OR FIBROSIS, OR BOTH, ARE ALSO COMMON MANIFESTATIONS OF THE CHRONIC PULMONARY REACTION. FEVER IS RARELY PROMINENT.
THE SEVERITY OF CHRONIC PULMONARY REACTIONS AND THEIR DEGREE OF RESOLUTION APPEAR TO BE RELATED TO THE DURATION OF THERAPY AFTER THE FIRST CLINICAL SIGNS APPEAR. PULMONARY FUNCTION MAY BE IMPAIRED PERMANENTLY, EVEN AFTER CESSATION OF THERAPY. THE RISK IS GREATER WHEN CHRONIC PULMONARY REACTIONS ARE NOT RECOGNIZED EARLY.
Hepatic
Neurologic
Dermatologic
Allergic
Gastrointestinal
Hematologic
Miscellaneous
Laboratory Adverse Events
OVERDOSAGE
DOSAGE & ADMINISTRATION
Adults
Pediatric Patients
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

NitrofurantoinNitrofurantoin CAPSULE
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!