Neuralgo Rheum
Neuralgo-Rheum 1.1ml Injection
FULL PRESCRIBING INFORMATION: CONTENTS*
- NEURALGO RHEUM DESCRIPTION
- NEURALGO RHEUM INDICATIONS AND USAGE
- NEURALGO RHEUM DOSAGE AND ADMINISTRATION
- NEURALGO RHEUM CONTRAINDICATIONS
- WARNINGS AND PRECAUTIONS
- NEURALGO RHEUM ADVERSE REACTIONS
- OVERDOSAGE
- CLINICAL PHARMACOLOGY
- DOSAGE
FULL PRESCRIBING INFORMATION
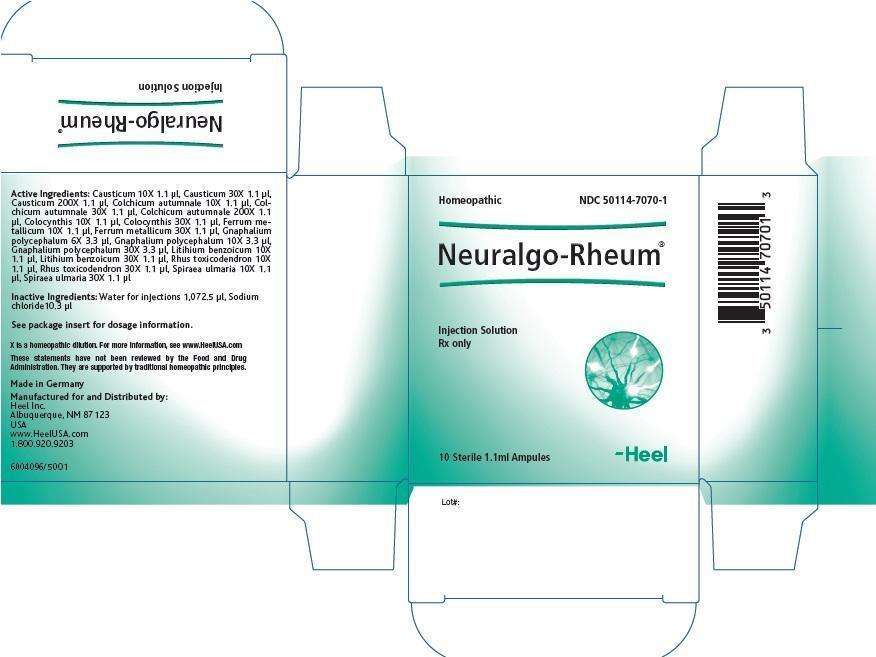
NEURALGO RHEUM DESCRIPTION
| Each 1.1 ml solution for injection ampule contains: | |||
| Active Ingredients: | |||
| Ingredient name | Potency | Quantity | Final dilution |
| Causticum | 10X | 1.1 µl | 12.99 |
| Causticum | 30X | 1.1 µl | 32.99 |
| Causticum | 200X | 1.1 µl | 203.00 |
| Colchicum autumnale | 10X | 1.1 µl | 12.99 |
| Colchicum autumnale | 30X | 1.1 µl | 32.99 |
| Colchicum autumnale | 200X | 1.1 µl | 203.00 |
| Colocynthis | 10X | 1.1 µl | 12.99 |
| Colocynthis | 30X | 1.1 µl | 32.99 |
| Ferrum metallicum | 10X | 1.1 µl | 12.99 |
| Ferrum metallicum | 30X | 1.1 µl | 32.99 |
| Gnaphalium polycephalum | 6X | 3.3 µl | 8.52 |
| Gnaphalium polycephalum | 10X | 3.3 µl | 12.52 |
| Gnaphalium polycephalum | 30X | 3.3 µl | 32.52 |
| Lithium benzoicum | 10X | 1.1 µl | 12.99 |
| Lithium benzoicum | 30X | 1.1 µl | 32.99 |
| Rhus toxicodendron | 10X | 1.1 µl | 12.99 |
| Rhus toxicodendron | 30X | 1.1 µl | 32.99 |
| Spiraea ulmaria | 10X | 1.1 µl | 12.99 |
| Spiraea ulmaria | 30X | 1.1 µl | 32.99 |
List of Excipients:
Water for injection 1,072.5 μl
Sodium Chloride 10.3 μl
NEURALGO RHEUM INDICATIONS AND USAGE
Neuralgo-Rheum® Injection Solution is a homeopathic drug product indicated for the treatment of nerve pain, soft tissue rheumatism and symptoms of disc protrusion.
NEURALGO RHEUM DOSAGE AND ADMINISTRATION
General Considerations
The dosage schedules listed below can be used as a general guide for the administration of Neuralgo-Rheum® Injection Solution.
• Neuralgo-Rheum® Injection Solution may be administered s.c., i.d., i.m., or i.v..
• The interval between injections is left to the discretion of the HCP, but should not exceed 1 ampule in 24 hours.
• Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
• Draw up required dose into syringe.
• Discard any unused ampule contents. Do not reuse ampule.
Standard Dosage:
Adults and children 12 years and older: 1 ml 1 to 3 times per 7 days.
Children 6 to 11 years: 0.7 ml 1 to 3 times per 7 days.
Children 2 to 5 years: 0.5 ml 1 to 3 times per 7 days.
Acute Dosage:
Adults and children 12 years and older: 1 ml daily, and then continue with standard dosage.
Children 6 to 11 years: 0.7 ml daily, and then continue with standard dosage.
Children 2 to 5 years: 0.5 ml daily, and then continue with standard dosage.
NEURALGO RHEUM CONTRAINDICATIONS
Neuralgo-Rheum® Injection Solution is contraindicated in patients with known hypersensitivity to Neuralgo-Rheum® or any of its ingredients.
WARNINGS AND PRECAUTIONS
Keep out of reach of children.
NEURALGO RHEUM ADVERSE REACTIONS
Post-marketing Experience
- No adverse events have been reported with a causal relationship to Neuralgo-Rheum® Injection Solution.
To report SUSPECTED ADVERSE REACTIONS, contact Heel Inc. at 1.800.920.9203
info@heelusa.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
No negative effects of an overdose have been reported and none are expected due to the homeopathic dilutions.
CLINICAL PHARMACOLOGY
Mechanism of Action
The exact mechanism of Neuralgo-Rheum® Injection Solution is not fully understood.
Pharmacodynamics
Not applicable for homeopathic medicinal products.
DOSAGE
1 ampule containing 1.1 ml solution for injection each containing the active ingredients in the strengths listed under Description.
Neuralgo RheumPSEUDOGNAPHALIUM OBTUSIFOLIUM, CAUSTICUM, COLCHICUM AUTUMNALE BULB, CITRULLUS COLOCYNTHIS FRUIT PULP, IRON, LITHIUM BENZOATE, TOXICODENDRON PUBESCENS LEAF and FILIPENDULA ULMARIA ROOT INJECTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||