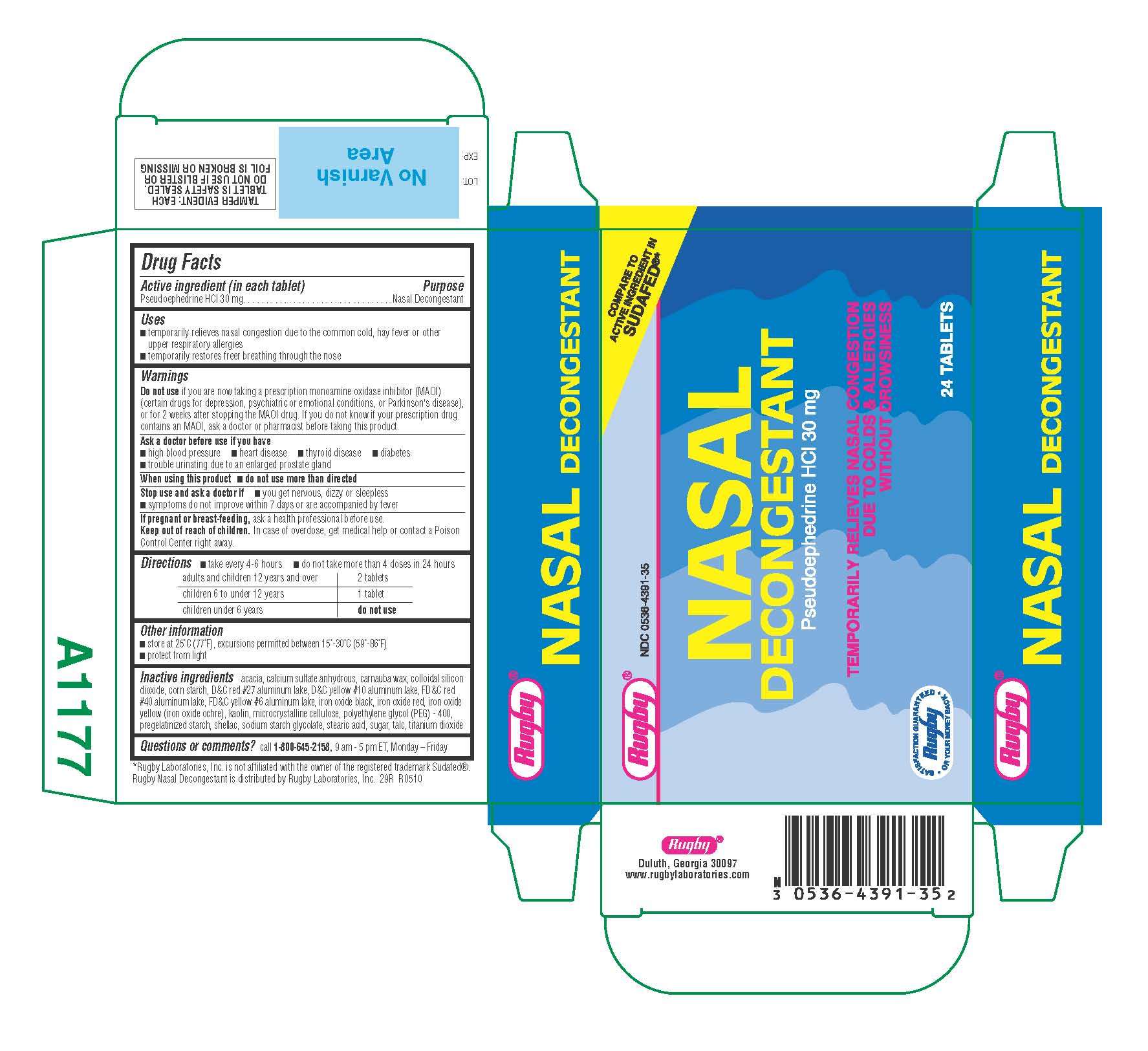
Nasal Decongestant
Rugby Laboratories, Inc
Time Cap Labs, Inc
DRUG FACTS
FULL PRESCRIBING INFORMATION
Active ingredient
Active ingredient (in each tablet)Purpose
PurposeUses
Uses- temporarily relieves nasal congestion due to the common cold, hay fever, or other upper respiratory allergies
- temporarily restores freer breathing through the nose
Do not use
Ask a doctor before use if you have
- high blood pressure
- heart disease
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
-
do not use more than directed
- you get nervous, dizzy or sleepless
- symptoms do not improve within 7 days or accompanied by fever
Keep out of reach of children.
Directions
- take every 4-6 hours
- do not take more than 4 doses in 24 hours
do not use
Other information
- store at 25°C (77°F), excursions permitted between 15°-30°C (59°-86°F)
- protect from light
Questions or comments? 1-800-645-2158
Nasal DecongestantPseudoephedrine HCl TABLET, SUGAR COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!