Naproxen Sodium
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- NAPROXEN SODIUM DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- INDICATIONS & USAGE
- NAPROXEN SODIUM CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- LABOR & DELIVERY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- NAPROXEN SODIUM ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
NAPROXEN SODIUM DESCRIPTION
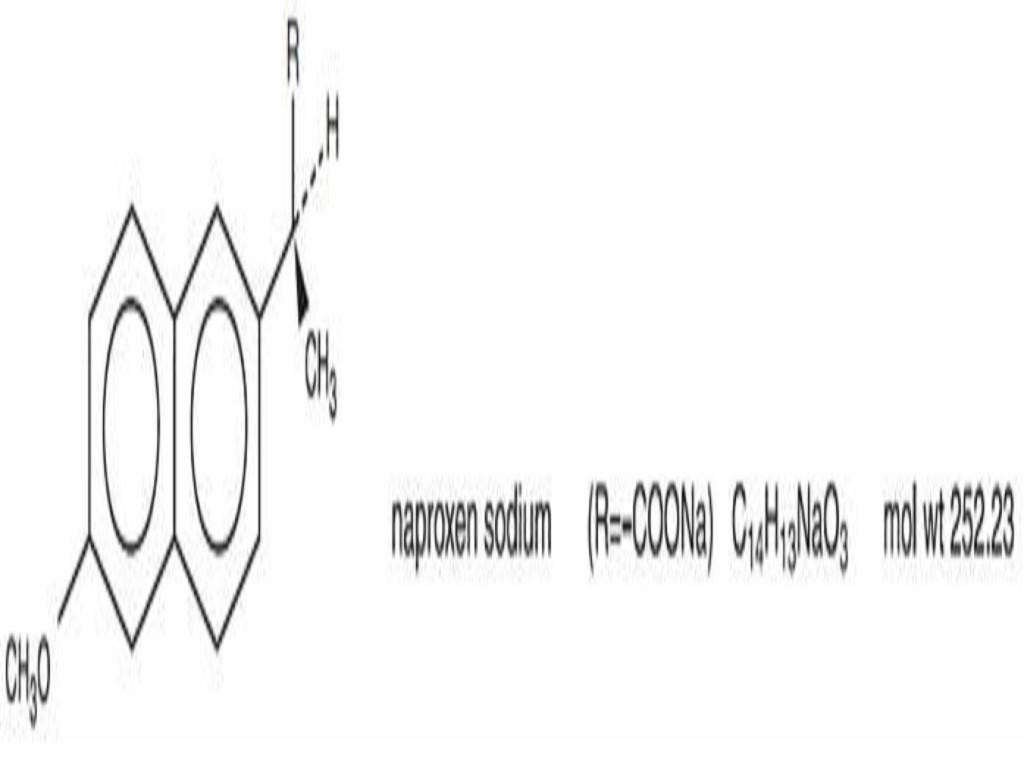
CLINICAL PHARMACOLOGY
PharmacodynamicsPharmacokinetics
PRECAUTIONS: Nursing Mothers
WARNINGS: Renal Effects
DOSAGE AND ADMINISTRATION
WARNINGS: Renal Effects
CLINICAL STUDIES
General InformationGeriatric Patients
INDICATIONS & USAGE
WARNINGSNAPROXEN SODIUM CONTRAINDICATIONS
WARNINGS: Anaphylactoid ReactionsPRECAUTIONS: Preexisting Asthma
WARNINGS
WARNINGS
CARDIOVASCULAR EFFECTSCardiovascular Thrombotic Events
Gastrointestinal Effects - Risk of Ulceration, Bleeding, and Perforation
CONTRAINDICATIONS
Gastrointestinal Effects- Risk of Ulceration, Bleeding, and Perforation
Renal Effects
WARNINGS: Advanced Renal Disease
Advanced Renal Disease
Anaphylactoid Reactions
CONTRAINDICATIONSPRECAUTIONS: Preexisting Asthma
Skin Reactions
Pregnancy
PRECAUTIONS
GeneralNaproxen-containing products such as Naproxen Sodium Tablets, USP, and other naproxen products should not be used concomitantly since they all circulate in the plasma as the naproxen anion.
Hepatic Effects
Hematological Effects
Preexisting Asthma:
INFORMATION FOR PATIENTS
Patients should be informed of the following information before initiating therapy with an NSAID and periodically during the course of ongoing therapy. Patients should also be encouraged to read the NSAID Medication Guide that accompanies each prescription dispensed.WARNINGS: Cardiovascular Effects
WARNINGS: Gastrointestinal Effects- Risk of Ulceration, Bleeding, and Perforation
WARNINGS
LABORATORY TESTS
DRUG INTERACTIONS
WARNINGS: Renal Effects
Other Information Concerning Drug Interactions
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
LABOR & DELIVERY
NURSING MOTHERS
PEDIATRIC USE
DOSAGE AND ADMINISTRATIONDOSAGE AND ADMINISTRATIONGERIATRIC USE
WARNINGS
WARNINGS: Renal Effects
NAPROXEN SODIUM ADVERSE REACTIONS
CLINICAL PHARMACOLOGY
Gastrointestinal (GI) Experiences, including:
Central Nervous System:
Dermatologic:
Special Senses:
Cardiovascular:
General:
Gastrointestinal (GI) Experiences, including:
General:
Body as a Whole:
Cardiovascular:
Gastrointestinal:
Hepatobiliary:
Hemic and Lymphatic:
Metabolic and Nutritional:
Nervous System:
Respiratory:
Dermatologic:
Special Senses:
Urogenital:
Reproduction (female):
Body as a Whole:
Cardiovascular:
Gastrointestinal:
Hepatobiliary:
Hemic and Lymphatic:
Metabolic and Nutritional:
Nervous System:
Respiratory:
Dermatologic:
Special Senses:
Urogenital:
OVERDOSAGE
DOSAGE & ADMINISTRATION
WARNINGSDifferent dose strengths and formulations (i.e., tablets, suspension) of the drug are not necessarily bioequivalent. The difference should be taken into consideration when changing formulation.
CLINICAL PHARMACOLOGY
WARNINGSPRECAUTIONS
Geriatric Patients
Patients With Moderate to Severe Renal Impairment
WARNINGS: Renal Effects
Rheumatoid Arthritis, Osteoarthritis and Ankylosing Spondylitis
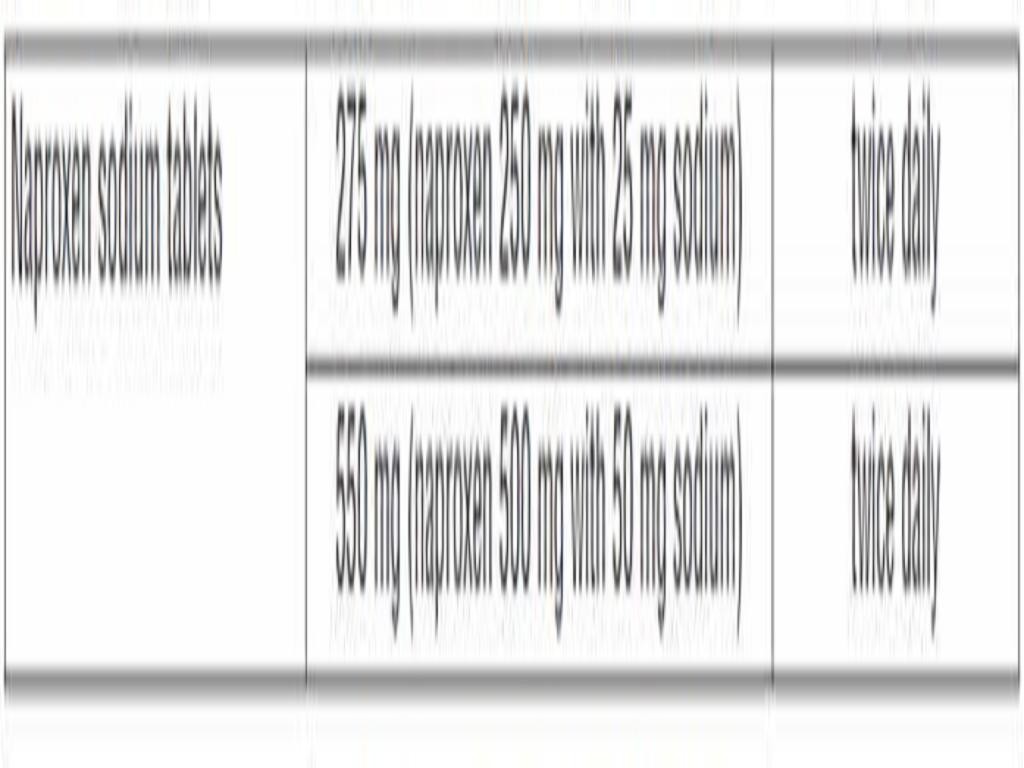
CLINICAL PHARMACOLOGY
Juvenile Arthritis
Management of Pain, Primary Dysmenorrhea, and Acute Tendonitis and Bursitis:
Acute Gout:
HOW SUPPLIED
Naproxen Sodium TabletsNaproxen Sodium Tablets,
STORAGE AND HANDLING
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Naproxen SodiumNaproxen Sodium TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!