Mononine
Coagulation Factor IX (Human) Mononine Monoclonal Antibody Purified
FULL PRESCRIBING INFORMATION: CONTENTS*
- MONONINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- CLINICAL STUDIES
- MONONINE INDICATIONS AND USAGE
- MONONINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- MONONINE ADVERSE REACTIONS
- MONONINE DOSAGE AND ADMINISTRATION
- STORAGE
- HOW SUPPLIED
- REFERENCES
- PRINCIPAL DISPLAY PANEL - 500 IU Carton
- PRINCIPAL DISPLAY PANEL - 1000 IU Carton
FULL PRESCRIBING INFORMATION
Rx only
MONONINE DESCRIPTION
Coagulation Factor IX (Human), Mononine® is a sterile, stable, lyophilized concentrate of Factor IX prepared from pooled human plasma and is intended for use in therapy of Factor IX deficiency, known as Hemophilia B or Christmas disease. Mononine® is purified of extraneous plasma-derived proteins, including Factors II, VII and X, by use of immunoaffinity chromatography. A murine monoclonal antibody to Factor IX is used as an affinity ligand to isolate Factor IX from the source material. Factor IX is then dissociated from the monoclonal antibody, recovered, purified further, formulated and provided as a sterile, lyophilized powder. The immunoaffinity protocol utilized results in a highly pure Factor IX preparation. It shows predominantly a single component by SDS polyacrylamide electrophoretic evaluation and has a specific activity of not less than 190 Factor IX units per mg total protein.
All Source Plasma used in the manufacture of this product was tested by FDA-licensed Nucleic Acid Testing (NAT) for HBV, HCV, and HIV-1 and found to be nonreactive (negative).
This concentrate has been processed by monoclonal antibody immunoaffinity chromatography during its manufacture, which has been shown to be capable of reducing the risk of viral transmission. Additionally, a chemical treatment protocol and nanofiltration used in its manufacture have also been shown to be capable of significant virus reductions. However, no procedure has been shown to be totally effective in removing the risk of viral infectivity from coagulation factor concentrates (see CLINICAL PHARMACOLOGY and WARNINGS ).
Mononine® is a highly purified preparation of Factor IX. When stored as directed, it will maintain its labeled potency for the period indicated on the container label.
Each vial contains the labeled amount of Factor IX activity expressed in International Units (IU). One IU represents the activity of Factor IX present in 1 mL of normal, pooled plasma. When reconstituted as recommended, the resulting solution is a clear, colorless, isotonic preparation of neutral pH, containing approximately 100 times the Factor IX potency found in an equal volume of plasma. Each mL of the reconstituted concentrate contains approximately 100 IU of Factor IX and non-detectable levels of Factors II, VII and X (<0.0025 IU per Factor IX unit using standard coagulation assays). Each vial also contains histidine (approx. 10mM), sodium chloride (approx. 0.066M), mannitol (approx. 3%) and polysorbate 80 (approx. 0.0075%). Hydrochloric acid and/or sodium hydroxide may have been used to adjust pH. Mononine® also contains trace amounts (≤50 ng mouse protein/100 Factor IX activity units) of the murine monoclonal antibody used in its purification (see CLINICAL PHARMACOLOGY ).
Mononine® is to be administered only intravenously.
CLINICAL PHARMACOLOGY
Hemophilia B, or Christmas disease, is an X-linked recessively inherited disorder of blood coagulation characterized by insufficient or abnormal synthesis of the clotting protein Factor IX. Factor IX is a vitamin K-dependent coagulation factor which is synthesized in the liver. Factor IX is activated by Factor XIa in the intrinsic coagulation pathway. Activated Factor IX (IXa), in combination with Factor VIII:C, activates Factor X to Xa, resulting ultimately in the conversion of prothrombin to thrombin and the formation of a fibrin clot. The infusion of exogenous Factor IX to replace the deficiency present in Hemophilia B temporarily restores hemostasis. Depending upon the subject's level of biologically active Factor IX, clinical symptoms range from moderate skin bruising or excessive hemorrhage after trauma or surgery to spontaneous hemorrhage into joints, muscles or internal organs including the brain. Severe or recurring hemorrhages can produce death, organ dysfunction or orthopedic deformity.
Infusion of Factor IX Complex concentrates that contain varying but significant amounts of the other liver-dependent blood coagulation proteins (Factors II, VII and X) into subjects with Hemophilia B, results in Factor IX recoveries ranging from approximately 0.57-1.1 IU/dL rise per IU/kg body weight infused with plasma half-lives for Factor IX ranging from approximately 23 hours to 31 hours.1,2 Infusion of Mononine® into ten subjects with severe or moderate Hemophilia B has shown a mean recovery of 0.67 IU/dL rise per IU/kg body weight infused and a mean half-life of 22.6 hours.3 After six months of experience with repeated infusions performed on the nine subjects who remained in the study, it was shown that the half-life and recovery was maintained at a level comparable to that found with the initial infusion. The six-month data showed a mean recovery of 0.68 IU/dL rise per IU/kg body weight infused and a mean half-life of 25.3 hours.3 The data show no statistically significant differences between the initial and six-month values.
Two studies were conducted to provide Mononine® for treatment of hemophilia B subjects who required extensive Factor IX replacement for surgery, trauma, or spontaneous bleeding (73 unique subjects and eight subjects enrolled twice for a total of 81 subjects), as well as to evaluate the safety and efficacy of Mononine®. The overall mean recovery during treatment was determined to be 1.23 ± 0.42 IU/dL rise/IU/kg (K) (range = 0.59 to 2.92 K) among the 55 subjects included in recovery analyses in the one study and to be 1.12 ± 0.52 K (range = 0.61 to 2.08 K) among 10 subjects included in these analyses in the second study. Five (5/81, 6%) subjects reported adverse events attributed to Mononine® across the two studies. In these studies, 100 doses of Mononine® were administered at what are considered high doses for a Factor IX concentrate, a range of 71 to 161 IU/kg to a total of 36 subjects. Sixty-seven (67) of these infusions were the subject of recovery analyses. Mean recovery tended to decrease as the dose of Mononine® increased: 1.09 ± 0.52 K at doses >75-95 IU/kg (n=38), 0.98 ± 0.45 K at doses >95-115 IU/kg (n=21), 0.70 ± 0.38 K at doses >115-135 IU/kg (n=2), 0.67 K at doses >135-155 IU/kg (n=1), and 0.73 ± 0.34 K at doses >155 IU/kg (n=5). Among the 36 subjects who received these high doses, only one (2.8%) reported an adverse experience with a possible relationship to Mononine® ("difficulty in concentrating"; subject recovered). In no subjects were thrombogenic complications observed or reported.4
The manufacturing procedure for Mononine® includes multiple processing steps that have been designed to reduce the risk of virus transmission. Validation studies of the monoclonal antibody (MAb) immunoaffinity chromatography/chemical treatment step and nanofiltration step used in the production of Mononine® document the virus reduction capacity of the processes employed. These studies were conducted using the relevant enveloped and non-enveloped viruses. The results of these virus validation studies utilizing a wide range of viruses with different physicochemical properties are summarized in Table 1 below:
| Manufacturing Step | Virus Reduction Factor (log10) | |||||
|---|---|---|---|---|---|---|
| Enveloped Viruses | Non-Enveloped Viruses | |||||
| HIV-1 | BVDV | PRV | WNV | HAV | Parvovirus 1 CPV, canine parvovirus, model for B19V (parvovirus B19) 2 PPV, porcine parvovirus, model for B19V |
|
| HIV, Human immunodeficiency virus type 1, model for HIV-1 and HIV-2 | ||||||
| BVDV, Bovine viral diarrhea virus, model for HCV | ||||||
| PRV, Pseudorabies virus, model for large enveloped DNA viruses, eg. herpes | ||||||
| WNV, West Nile virus | ||||||
| HAV, Hepatitis A virus | ||||||
| MAb Chromatography | ≥5.8 | 5.8 | ≥8.3 | ≥6.5 | [3.3] |
6.61 |
| Nanofiltration | ≥4.4 |
≥6.8 |
≥7.8 |
≥6.3 |
≥6.7 |
≥8.22,
 |
| Cumulative Virus Reduction (log10) | ≥10.2 | ≥12.6 | ≥16.1 | ≥12.8 | ≥6.7 | ≥14.8 |
CLINICAL STUDIES
The virus safety of Mononine® has been studied in clinical trials of two cohorts of hemophilia B subjects previously unexposed to blood or blood products.5 One cohort of subjects included those with moderate to severe factor IX deficiency requiring chronic replacement therapy (41 subjects were dosed); the second cohort included subjects with a mild deficiency requiring factor IX replacement for surgical procedures (10 subjects were dosed).
These subjects were followed for serum alanine aminotransferase (ALT) elevations, as well as for a range of viral serologies. Thirty-seven (37) subjects (30 with moderate to severe deficiency and seven with a mild deficiency) were evaluable for assessment of virus hepatitis safety by the International Society on Thrombosis and Haemostasis-Scientific and Standardization Committee criteria. None of these subjects showed evidence of transmission of hepatitis A, B, C, or HIV.
Mononine® contains trace amounts of the murine monoclonal antibody (MAb) used in its purification (≤50 ng mouse protein/100 IU). While the levels of mouse protein are extremely low, infusion of such proteins might theoretically induce human anti-mouse antibody (HAMA) responses. To test this possibility, human IgG, IgM, and IgE antibodies to mouse IgG were assessed by immunoradiometric assay (IRMA) in 11 hemophilia B subjects who received Mononine® and were previously untreated with other blood products. HAMAs were evaluated prior to the first infusion and at 2 to 42 months after initial treatment. Human IgE antibodies to mouse IgG were below the level of detectability at all time points for all subjects, and there were no statistically significant increases in either human IgG antibodies or human IgM antibodies to mouse protein.6
In clinical studies of Mononine® subjects were monitored for evidence of disseminated intravascular coagulation. In six subjects evaluated after infusion, fibrinogen levels and platelet counts were unchanged, and fibrin degradation products did not appear.3
In further clinical evaluations of Mononine® in a crossover study with a Factor IX Complex concentrate, Mononine® was not associated with the formation of prothrombin activation fragment (F1+2) whereas the Factor IX Complex was associated with the formation of prothrombin activation fragment (F1+2).3,7 Prothrombin activation fragment (F1+2) is indicative of activation of prothrombin.
During the period from 1992 to 1996, five subjects showed transient ALT elevations that were greater than twice the upper normal limit. These subjects were investigated thoroughly and none of the ALT elevations was associated with seroconversion. In three of the five subjects, a single ALT elevation greater than 2 times the upper limit of normal was recorded during the course of the study. No concomitant symptoms occurred and the virus hepatitis serology tests did not reveal any abnormalities. In addition, in one of these three subjects with single ALT elevations, a relationship to Mononine® could be excluded due to a span of 18 months between the infusion of Mononine® and occurrence of the elevated ALT level. In one of the two remaining subjects, the ALT level had been elevated prior to the first infusion of Mononine® and normalized thereafter. Subsequently, this subject's ALT levels were elevated intermittently over a period of 24 months, which appeared to be temporally related to the administration of concomitant medications: acetaminophen, amoxicillin, cephalosporins and halothane. These medications are known to cause liver enzyme elevations. Further, there were no clinical signs of viral hepatitis, nor any other viral disease in these four subjects. The remaining subject of the five was found to have recurring ALT elevations that persisted for a period of five months, gradually decreasing to normal levels. Approximately three days after his first Mononine® infusion this subject received hepatitis B immune globulin and his first injection of hepatitis B vaccine. At that time, the subject's ALT level was slightly above the upper limit of normal (55 IU/L, upper limit of normal: 35). Five days later, the subject experienced flu-like symptoms, nausea and vomiting, which were treated with ampicillin and promethazine. The ALT value recorded eight days thereafter (approximately 13 days after the Mononine® infusion) was found to be clearly elevated at 629 IU/L. ALT levels subsequently decreased again and were in the range of 160 to 220 IU/L for the next four to five months, with mildly elevated aspartate aminotransferase and creatinine phosphokinase values. Serology for hepatitis A, B, and C remained negative (except for the expected positive serology of anti-HBs due to the vaccination against hepatitis B). As a result, there was no serological evidence of hepatitis A, B, or C. This subject's idiosyncratic spikes in aminotransferase values and gastrointestinal symptoms were not considered to be of viral origin. However, a causal relationship between prior administration of Mononine® and these aminotransferase elevations and mild symptoms could not be ruled out.
MONONINE INDICATIONS AND USAGE
Mononine® is indicated for the prevention and control of bleeding in Factor IX deficiency, also known as Hemophilia B or Christmas disease.
Mononine® is not indicated in the treatment or prophylaxis of Hemophilia A patients with inhibitors to Factor VIII.
Mononine® contains non-detectable levels of Factors II, VII and X (<0.0025 IU per Factor IX unit using standard coagulation assays) and is, therefore, not indicated for replacement therapy of these clotting factors.
Mononine® is also not indicated in the treatment or reversal of coumarin-induced anticoagulation or in a hemorrhagic state caused by hepatitis-induced lack of production of liver dependent coagulation factors.
MONONINE CONTRAINDICATIONS
Known hypersensitivity to mouse protein is a contraindication to Mononine®.
WARNINGS
Mononine® is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, that can cause disease. Because Mononine® is made from human blood, it may carry a risk of transmitting infectious agents, e.g., viruses, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current viral infections and by inactivating and/or removing certain viruses during manufacture (see DESCRIPTION section for virus reduction measures). The manufacturing procedure for Mononine® includes processing steps designed to reduce further the risk of virus transmission. Stringent procedures, utilized at plasma collection centers, plasma testing laboratories, and fractionation facilities are designed to reduce the risk of virus transmission. The primary virus reduction step of the Mononine® manufacturing process is the use of a nanofilter designed to separate viruses from Factor IX. In addition, the purification procedure (several chromatography steps) used in the manufacture of Mononine® also provides virus reduction capacity. Despite these measures, such products may still potentially contain human pathogenic agents, including those not yet known or identified. Thus the risk of transmission of infectious agents cannot be totally eliminated. Any infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to CSL Behring at 1-866-915-6958 (in the U.S. and Canada). The physician should discuss the risks and benefits of this product with the patient.
Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly nonA, nonB hepatitis. (See Information For Patients .)
Since the use of Factor IX Complex concentrates has historically been associated with the development of thromboembolic complications, the use of Factor IX-containing products may be potentially hazardous in patients with signs of fibrinolysis and in patients with disseminated intravascular coagulation (DIC).
Hypersensitivity and allergic type hypersensitivity reactions, including anaphylaxis, have been reported for all factor IX products. Frequently, these events have occurred in close temporal association with the development of factor IX inhibitors. Patients should be informed of the early symptoms and signs of hypersensitivity reactions, including hives, generalized urticaria, angioedema, chest tightness, dyspnea, wheezing, faintness, hypotension, tachycardia, and anaphylaxis. Patients should be advised to discontinue use of product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if any of these symptoms occur.
Preliminary information suggests a relationship may exist between the presence of major deletion mutations in the factor IX gene and an increased risk of inhibitor formation and of acute hypersensitivity reactions. Patients known to have major deletion mutations of the factor IX gene should be observed closely for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of initial exposure to product.
Nephrotic syndrome has been reported following attempted immune tolerance induction with factor IX products in Hemophilia B patients with factor IX inhibitors and a history of severe allergic reactions to factor IX. The safety and efficacy of using Mononine® in attempted immune tolerance induction has not been established.
PRECAUTIONS
Extensive clinical experience suggests that there is a lower risk of thromboembolic complications with the use of Mononine® than with prothrombin complex concentrates. However, as with all products containing Factor IX, caution should be exercised when administering Mononine® to patients with liver disease, to patients post-operatively, to neonates, or to patients at risk of thromboembolic phenomena or DIC.8,9 In each of these situations, the potential benefit of treatment with Mononine® should be weighed against the potential risk of these complications.
Mononine® should be administered intravenously at a rate that will permit observation of the patient for any immediate reaction. Rates of infusion of up to 225 IU per minute have been regularly tolerated with no adverse reactions. If any reaction takes place that is thought to be related to the administration of Mononine®, the rate of infusion should be decreased or the infusion stopped, as dictated by the response of the patient. The infusion should be stopped promptly and appropriate countermeasures and supportive therapy should be administered should evidence of an acute hypersensitivity reaction be observed. Patients known to have major deletion mutations of the factor IX gene may be at increased risk for inhibitor formation and acute hypersensitivity reactions. (See WARNINGS .)
During the course of treatment, determination of daily Factor IX levels is advised to guide the dose to be administered and the frequency of repeated infusions. Individual patients may vary in their response to Mononine®, achieving different levels of in vivo recovery and demonstrating different half-lives.
The use of high doses of Factor IX Complex concentrates has been reported to be associated with instances of myocardial infarction, disseminated intravascular coagulation, venous thrombosis and pulmonary embolism. Generally a Factor IX level of 25-50% [IU/dL] is considered adequate for hemostasis, including major hemorrhages and surgery. Attempting to maintain Factor IX levels of >75-100% [IU/dL] during treatment is not routinely recommended nor required. To achieve Factor IX levels that will remain above 25% [IU/dL] between once a day administrations, each daily dose should attempt to raise the 30-minute post-infusion Factor IX level to 50-60% [IU/dL] (see DOSAGE AND ADMINISTRATION ).
No controlled studies have been available regarding the use of ε-amino caproic acid or other antifibrinolytic agents following an initial infusion of Mononine® for the prevention or treatment of oral bleeding following trauma or dental procedures such as extractions.
Information For Patients
Patients should be informed of the early symptoms and signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, dyspnea, wheezing, faintness, hypotension, and anaphylaxis. Patients should be advised to discontinue use of the product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if these symptoms occur.
Some viruses such as hepatitis A are particularly difficult to remove or inactivate at this time. Although the overwhelming number of hepatitis A cases are community acquired, there have been reports of these infections associated with the use of some plasma-derived products. Therefore, physicians should be alert to the potential symptoms of hepatitis A infections and inform patients under their supervision receiving plasma-derived products to report potential symptoms promptly.
Evidence of hepatitis A may include several days to weeks of poor appetite, tiredness, and low-grade fever followed by nausea, vomiting and pain in the belly. Dark urine and a yellowed complexion are also common symptoms. Patients should be encouraged to consult their physicians if such symptoms occur.
Pregnancy Category C
Animal reproduction studies have not been conducted with Mononine®. It is also not known whether Mononine® can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Mononine® should be given to a pregnant woman only if clearly needed.
Pediatric Use
Evaluation of the safety and effectiveness of Mononine® treatment in 51 pediatric patients between the ages of 1 day and 20 years, as a part of virus safety trials and trials for surgery, trauma or spontaneous bleeding, showed that excellent hemostasis was achieved with no thrombotic complications.10 Included in the experience with patients aged birth to 20 years are two long-term virus safety studies demonstrating lack of virus transmission. Dosing in children is based on body weight and is generally based on the same guidelines as for adults (see DOSAGE AND ADMINISTRATION ).
Geriatric Use
Clinical studies of Mononine® did not include sufficient numbers of patients aged 65 and over to determine whether they respond differently from younger patients. As for all patients, dosing for geriatric patients should be appropriate to their overall situation.
MONONINE ADVERSE REACTIONS
As with the intravenous administration of other plasma-derived products, the following reactions may be observed following administration: headache, fever, chills, flushing, nausea, vomiting, tingling, lethargy, hives, stinging or burning at the infusion site or manifestations of allergic reactions. In a clinical study with Mononine® in previously untreated hemophilia B patients, five patients experienced ALT elevations. Serologic tests for hepatitis A, hepatitis B, hepatitis C, Cytomegalovirus, and Epstein-Barr virus were negative.
The following adverse reactions have been spontaneously reported during post-marketing use of Mononine® as well as other Factor IX products: anaphylaxis, angioedema, cyanosis, dyspnea, hypotension, thrombosis, inadequate therapeutic response, and inhibitor development.
There is a potential risk of thromboembolic episodes following the administration of Mononine® (see WARNINGS and PRECAUTIONS ).
The patient should be monitored closely during the infusion of Mononine® to observe for the development of any reaction. If any reaction takes place that is thought to be related to the administration of Mononine®, the rate of infusion should be decreased or the infusion stopped, as dictated by the response of the patient.
Should evidence of an acute hypersensitivity reaction be observed, the infusion should be stopped promptly and appropriate countermeasures and supportive therapy should be administered.
MONONINE DOSAGE AND ADMINISTRATION
Mononine® is intended for intravenous administration only. It should be reconstituted with the volume of Sterile Water for Injection, USP supplied with the lot, and administered within three hours of reconstitution. Do not refrigerate after reconstitution. After administration, any unused solution and the administration equipment should be discarded.
As a general rule, 1 IU of Factor IX activity per kg can be expected to increase the circulating level of Factor IX by 1% [IU/dL] of normal. The following formula provides a guide to dosage calculations:
| Number of Factor IX | = | Body Weight | × | desired Factor IX | × | 1.0 IU/kg |
| IU required (IU) | (in kg) | increase (% or IU/dL normal) | [per IU/dL] | |||
The amount of Mononine® to be infused, as well as the frequency of infusions, will vary with each patient and with the clinical situation.11,12
As a general rule, the level of Factor IX required for treatment of different conditions is as follows:
| Minor Spontaneous Hemorrhage, Prophylaxis | Major Trauma or Surgery | |
|---|---|---|
| Desired levels of Factor IX for Hemostasis | 15-25% [or IU/dL] |
25-50% [or IU/dL] |
| Initial loading dose to achieve desired level | up to 20-30 IU/kg | up to 75 IU/kg |
| Frequency of dosing | once; repeated in 24 hours if necessary | every 18-30 hours, depending on T1/2 and measured Factor IX levels |
| Duration of treatment | once; repeated if necessary | up to ten days, depending upon nature of insult |
Recovery of the loading dose varies from patient to patient. Doses administered should be titrated to the patient's response. Mononine® administered in doses of ≥75 IU/kg were well tolerated (see CLINICAL PHARMACOLOGY ).
In the presence of an inhibitor to Factor IX, higher doses of Mononine® might be necessary to overcome the inhibitor (see PRECAUTIONS ). No data on the treatment of patients with inhibitors to Factor IX with Mononine® are available.
For information on rate of administration, see Rate of Administration , below.
Reconstitution
- Warm both the diluent and Mononine® in unopened vials to room temperature [not above 37°C (98°F)].
- Remove the caps from both vials to expose the central portions of the rubber stoppers.
- Treat the surface of the rubber stoppers with antiseptic solution and allow them to dry.
- Using aseptic technique, insert one end of the double-end needle into the rubber stopper of the diluent vial. Invert the diluent vial and insert the other end of the double-end needle into the rubber stopper of the Mononine® vial. Direct the diluent, which will be drawn in by vacuum, over the entire surface of the Mononine® cake. (In order to assure transfer of all the diluent, adjust the position of the tip of the needle in the diluent vial to the inside edge of the diluent stopper.) Rotate the vial to ensure complete wetting of the cake during the transfer process.
- Remove the diluent vial to release the vacuum, then remove the double-end needle from the Mononine® vial.
- Gently swirl the vial until the powder is dissolved and the solution is ready for administration. The concentrate routinely and easily reconstitutes within one minute. To assure sterility, Mononine® should be administered within three hours after reconstitution.
- Product should be filtered prior to use as described under Administration. Parenteral drug preparations should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Administration
Intravenous Injection
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Plastic disposable syringes are recommended with Mononine® solution. The ground glass surfaces of all-glass syringes tend to stick with solutions of this type. Please note, this concentrate is supplied with a SELF-VENTING filter spike.
- Using aseptic technique, attach the vented filter spike to a sterile disposable syringe.
CAUTION: The use of other, non-vented filter needles or spikes without the proper procedure may result in an air lock and prevent the complete transfer of the concentrate.
CAUTION: DO NOT INJECT AIR INTO THE MONONINE® VIAL. The self-venting feature of the vented filter spike precludes the need to inject air in order to facilitate withdrawal of the reconstituted solution. The injection of air could cause partial product loss through the vent filter. - Insert the vented filter spike into the stopper of the Mononine® vial, invert the vial, and position the filter spike so that the orifice is at the inside edge of the stopper.
- Withdraw the reconstituted solution into the syringe.
- Discard the filter spike. Perform venipuncture using the enclosed winged needle with microbore tubing. Attach the syringe to the luer end of the tubing.
CAUTION: Use of other winged needles without microbore tubing, although compatible with the concentrate, will result in a larger retention of solution within the winged infusion set.
Rate of Administration
The rate of administration should be determined by the response and comfort of the patient; intravenous dosage administration rates of up to 225 IU/minute have been regularly tolerated without incident. When reconstituted as directed, i.e., to approximately 100 IU/mL, Mononine® should be administered at a rate of approximately 2.0 mL per minute.
STORAGE
When stored at refrigerator temperature, 2-8°C (36-46°F), Mononine® is stable for the period indicated by the expiration date on its label. Within this period, Mononine® may be stored at room temperature not to exceed 25°C (77°F), for up to one month.
Avoid freezing, which may damage container for the diluent.
HOW SUPPLIED
Mononine® is supplied in a single dose vial with Sterile Water for Injection, USP, double-ended needle for reconstitution, vented filter spike for withdrawal, winged infusion set and alcohol swabs. Factor IX activity in IU is stated on the label of each vial.
Each product package consists of the following:
| NDC Number | Component | Approximate FIX Activity (IU) |
|---|---|---|
| 0053-6232-02 | Carton (kit) containing one vial of Mononine® [NDC 0053-6242-01], one 5 mL vial of Sterile Water for Injection, USP (diluent) [NDC 0053-7653-06], one double-ended needle for reconstitution, one vented filter spike for withdrawal, one winged infusion set, and alcohol swabs. | 500 (MID) |
| 0053-6233-02 | Carton (kit) containing one vial of Mononine® [NDC 0053-6243-01], one 10 mL vial of Sterile Water for Injection, USP (diluent) [NDC 0053-7653-11], one double-ended needle for reconstitution, one vented filter spike for withdrawal, one winged infusion set, and alcohol swabs. | 1000 (HIGH) |
REFERENCES
- Zauber NP, Levin J. Factor IX levels in patients with hemophilia B (Christmas disease) following transfusion with concentrates of Factor IX or fresh frozen plasma (FFP). Medicine (Baltimore) 56(3):213-24, 1977.
- Smith KJ, Thompson AR. Labeled Factor IX Kinetics in Patients with Hemophilia-B. Blood 58(3):625-629, 1981.
- Kim HC, McMillan CW, White GC, Bergman GE, Horton MW, Saidi P. Purified Factor IX Using Monoclonal Immunoaffinity Technique: Clinical Trials in Hemophilia B and Comparison to Prothrombin Complex Concentrates. Blood 79(3):568-575, 1992.
- Warrier I, Kasper CK, White II GC, Shapiro AD, Bergman GE, the Mononine® Study Group. Safety of high doses of a monoclonal antibody-purified factor IX concentrate. Am J Hematol 49:92-94, 1995.
- Shapiro AD, Ragni MV, Lusher JM, Culbert S, Koerper MA, Bergman GE, Hannan MM. Safety and Efficacy of Monoclonal Antibody Purified Factor IX Concentrate in Previously Untreated Patients with Hemophilia B. Thrombosis and Haemostasis 75:30-35, 1996.
- Davis HM, Nash DW, Clymer MD, Frigo ML, Bergman GE. Lack of immune response to mouse IgG in previously untreated haemophilia A and haemophilia B patients treated with monoclonal antibody purified factor VIII and factor IX preparations. Haemophilia 3(2):102-107, April 1997.
- Kim HC, Matts L, Eisele J, Czachur M, Saidi P. Monoclonal Antibody Purified Factor IX - Comparative Thrombogenicity to Prothrombin Complex Concentrate. Seminars in Hematology 28 (Suppl. 6 to no. 3):15-20, July 1991.
- Aledort LM: Factor IX and Thrombosis. Scand J Haematology Suppl. 30:40, 1977.
- Cederbaum AI, Blatt PM, Roberts HR. Intravascular coagulation with use of human prothrombin complex concentrates. Ann Intern Med 84:683-687, 1976.
- Kurczynski E, Lusher JM, Pitel P, Shapiro AD, Bergman GE, the Mononine® Study Group. Safety and efficacy of monoclonal antibody-purified factor IX concentrate for management of bleeding and surgical prophylaxis in previously treated children with hemophilia B. Int J Ped Hemat/Oncol 2:211-216, 1995.
- Kasper CK, Dietrich SL. Comprehensive Management of Hemophilia. Clin Haematol 14(2):489-512, 1985.
- Johnson AJ, Aronson DL, Williams WJ. Preparation and clinical use of plasma and plasma fractions. Chap 167 in Hematology 3rd Edition, Williams WJ, Beutler E, Erslev AJ, Lichtman MA (Eds.), McGraw Hill Book Co. New York: pp 1563-1583, 1983.
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767
Revised February, 2013
13925-07
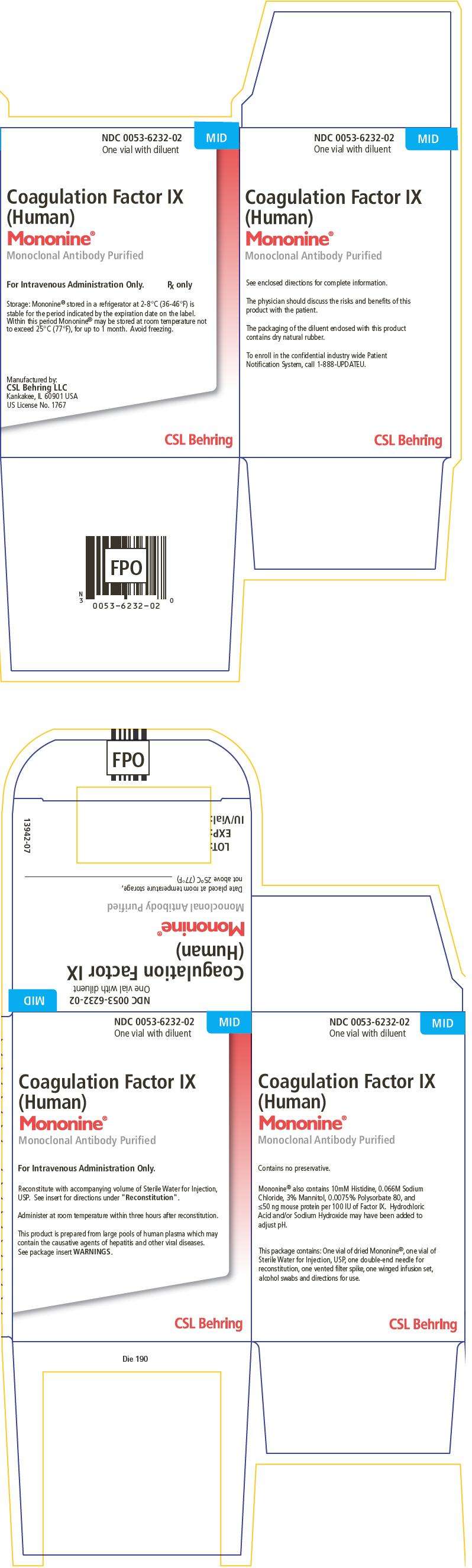
PRINCIPAL DISPLAY PANEL - 500 IU Carton
NDC 0053-6232-02
One vial with diluent
MID
Coagulation Factor IX
(Human)
Mononine®
Monoclonal Antibody Purified
For Intravenous Administration Only.
Rx only
Storage: Mononine® stored in a refrigerator at 2-8°C (36-46°F) is
stable for the period indicated by the expiration date on the label.
Within this period Mononine® may be stored at room temperature not
to exceed 25°C (77°F), for up to 1 month. Avoid freezing.
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767
CSL Behring
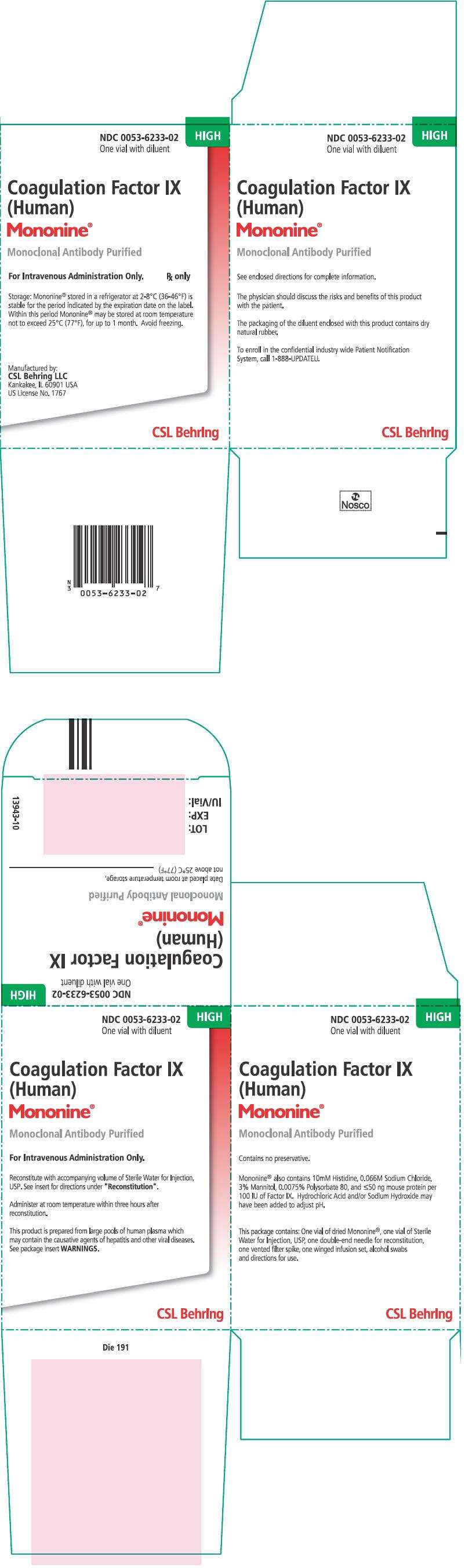
PRINCIPAL DISPLAY PANEL - 1000 IU Carton
NDC 0053-6233-02
One vial with diluent
HIGH
Coagulation Factor IX
(Human)
Mononine®
Monoclonal Antibody Purified
For Intravenous Administration Only.
Rx only
Storage: Mononine® stored in a refrigerator at 2-8°C (36-46°F) is
stable for the period indicated by the expiration date on the label.
Within this period Mononine® may be stored at room temperature
not to exceed 25°C (77°F), for up to 1 month. Avoid freezing.
Manufactured by:
CSL Behring LLC
Kankakee, IL 60901 USA
US License No. 1767
CSL Behring
Mononinecoagulation factor IX human KIT
| ||||||||||||||||||||||||||||||||||||||||
Mononinecoagulation factor IX human KIT
| ||||||||||||||||||||||||||||||||||||||||