MIRTAZAPINE
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MIRTAZAPINE DESCRIPTION
- CLINICAL PHARMACOLOGY
- GERIATRIC USE
- CLINICAL STUDIES
- INDICATIONS & USAGE
- MIRTAZAPINE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- MIRTAZAPINE ADVERSE REACTIONS
- DRUG ABUSE AND DEPENDENCE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Anyone considering the use of mirtazapine orally disintegrating tablets or any other antidepressant in a child, adolescent, or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Mirtazapine is not approved for use in pediatric patients. (See Warnings: Clinical Worsening and Suicide Risk, Precautions: Information for Patients, and Precautions: Pediatric Use)
MIRTAZAPINE DESCRIPTION
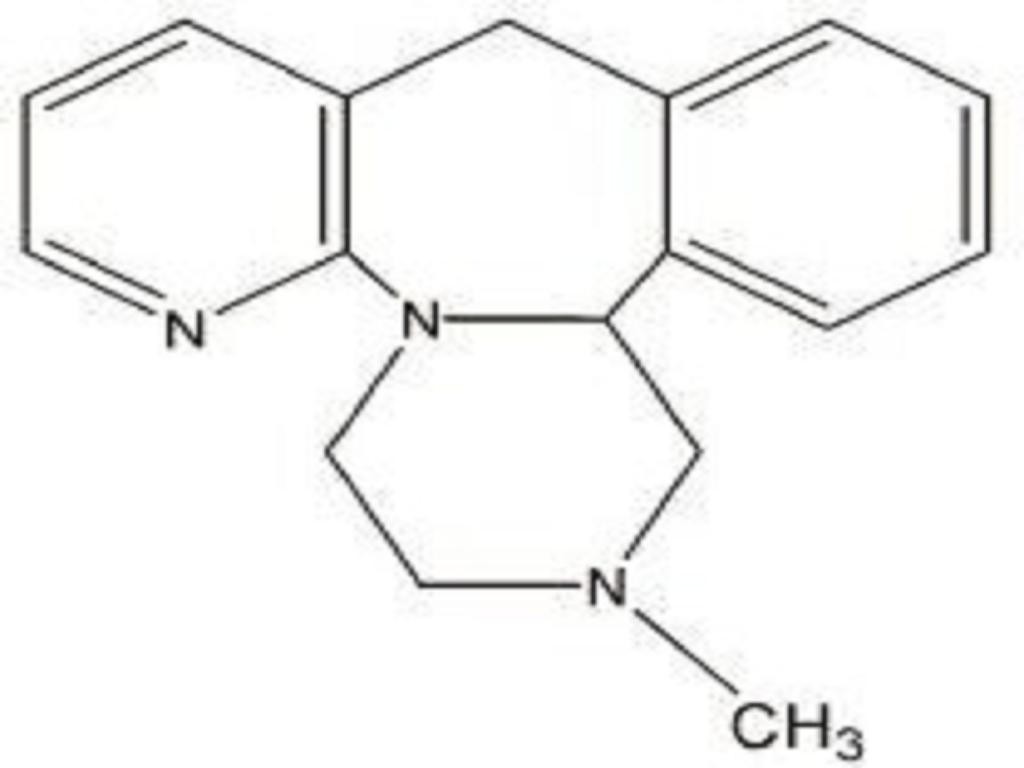
CLINICAL PHARMACOLOGY
2
2 31A1B
1
1
In vitro
GERIATRIC USE
PRECAUTIONSDOSAGE AND ADMINISTRATION
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
CLINICAL STUDIES
INDICATIONS & USAGE
CLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
MIRTAZAPINE CONTRAINDICATIONS
WARNINGS
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers.
PRECAUTIONS
Information for Patients
PRECAUTIONS: Pediatric Use
CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
22CLINICAL PHARMACOLOGYDOSAGE AND ADMINISTRATION
INFORMATION FOR PATIENTS
CLINICAL PHARMACOLOGYIn vitroin vitro
2
in vitro in vitro in vivo
2
PREGNANCY
Pregnancy Category C
22
NURSING MOTHERS
PEDIATRIC USE
PRECAUTIONS-Increased Appetite/Weight Gain
MIRTAZAPINE ADVERSE REACTIONS
WARNINGSPRECAUTIONS
Body as a Whole:frequent:infrequent: rare:
Cardiovascular System:frequent: infrequent:rare:
Digestive System:frequent: infrequent: rare:
Endocrine System:rare:
Hemic and Lymphatic System: rare:
Metabolic and Nutritional Disorders:frequent: infrequent: rare:
Musculoskeletal System:frequent: infrequent:rare:
Nervous System:frequent: infrequent: rare:
Respiratory System:frequent: infrequent: rare:
Skin and Appendages:frequent: infrequent: rare:
Special Senses:infrequent: rare:
Urogenital System:frequent:infrequent: rare:
DRUG ABUSE AND DEPENDENCE
OVERDOSAGE
Physicians’ Desk Reference (
DOSAGE & ADMINISTRATION
PRECAUTIONSCLINICAL PHARMACOLOGY
CLINICAL PHARMACOLOGY
HOW SUPPLIED
30 mg Tablets –
Storage
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MIRTAZAPINEMIRTAZAPINE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!