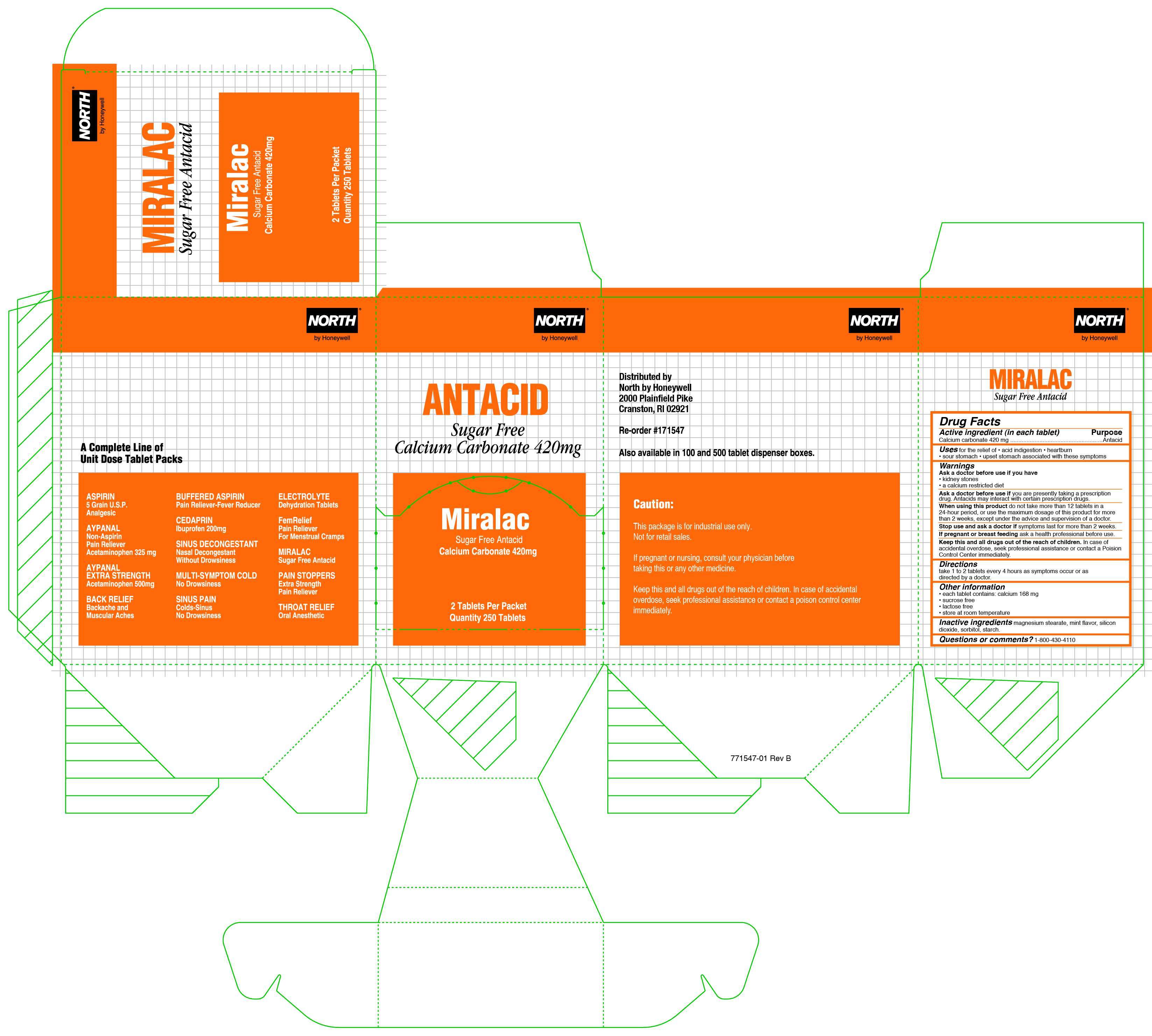
MIRALAC ANTACID
MIRALAC
FULL PRESCRIBING INFORMATION: CONTENTS*
- Active ingredient (in each tablet)
- Purpose
- MIRALAC ANTACID Uses
- Warnings
- Directions
- MIRALAC ANTACID Other information
- Inactive ingredients
- Questions or comments?
- Principal Display Panel
FULL PRESCRIBING INFORMATION
Active ingredient (in each tablet)
Calcium carbonate 420 mg
Purpose
Antacid
MIRALAC ANTACID Uses
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Warnings
Enter section text here
Ask a doctor before use if you have
- kidney stones
- calcium restricted diet
Ask a doctor before use if
you are presently taking a prescription drug. Antacids may interact with certain prescription drugs
Stop use and ask a doctor if
symptoms last for more than 2 weeks
ask a health professional before use
Keep this and all other drugs out of the reach of children
In case of accidental overdose, seek professional assistance or contact a Poison Control Center immediately.
Directions
take 1 to 2 tablets every 4 hours as symptoms occur or as directed by a doctor.
MIRALAC ANTACID Other information
each tablet contains: calcium 168 mg
sucrose free
lactose free
store at room temperature
Inactive ingredients
magnesium stearate, mint flavor, silicon dioxide, sorbitol, starch
Questions or comments?
1-800-430-4110
Principal Display Panel
 MM1
MM1
MIRALAC ANTACIDCALCIUM CARBONATE TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!