Metronidazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METRONIDAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METRONIDAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- GERIATRIC USE
- PEDIATRIC USE
- METRONIDAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
METRONIDAZOLE DESCRIPTION
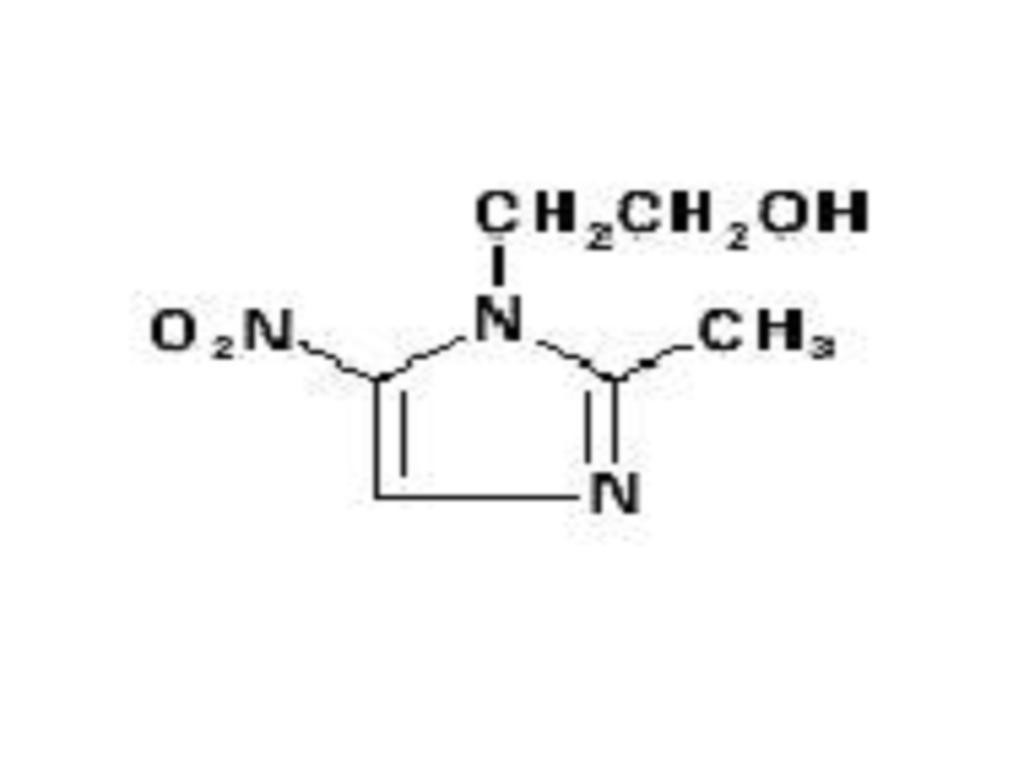
CLINICAL PHARMACOLOGY
Microbiology
Susceptibility Tests
INDICATIONS & USAGE
Symptomatic TrichomoniasisAsymptomatic Trichomoniasis
Treatment of Asymptomatic Consorts
Amebiasis
Anaerobic Bacterial Infections
METRONIDAZOLE CONTRAINDICATIONS
WARNINGS
Convulsive Seizures and Peripheral NeuropathyPRECAUTIONS
GeneralINFORMATION FOR PATIENTS
LABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category B
Use of metronidazole for trichomoniasis during pregnancy should be restricted to those in whom alternative treatment has been inadequate. Use of metronidazole for trichomoniasis in the first trimester of pregnancy should be carefully evaluated because metronidazole crosses the placental barrier and its effects on the human fetal organogenesis are not known (see above).
NURSING MOTHERS
GERIATRIC USE
PEDIATRIC USE
METRONIDAZOLE ADVERSE REACTIONS
OVERDOSAGE
Treatment
DOSAGE & ADMINISTRATION
Trichomoniasis
In the Female
In the Male
Amebiasis
Adults
Pediatric Patients
Anaerobic Bacterial Infections
HOW SUPPLIED
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MetronidazoleMetronidazole TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!