Metronidazole
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- METRONIDAZOLE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METRONIDAZOLE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- DRUG & OR LABORATORY TEST INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- GERIATRIC USE
- PEDIATRIC USE
- METRONIDAZOLE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- REFERENCES
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
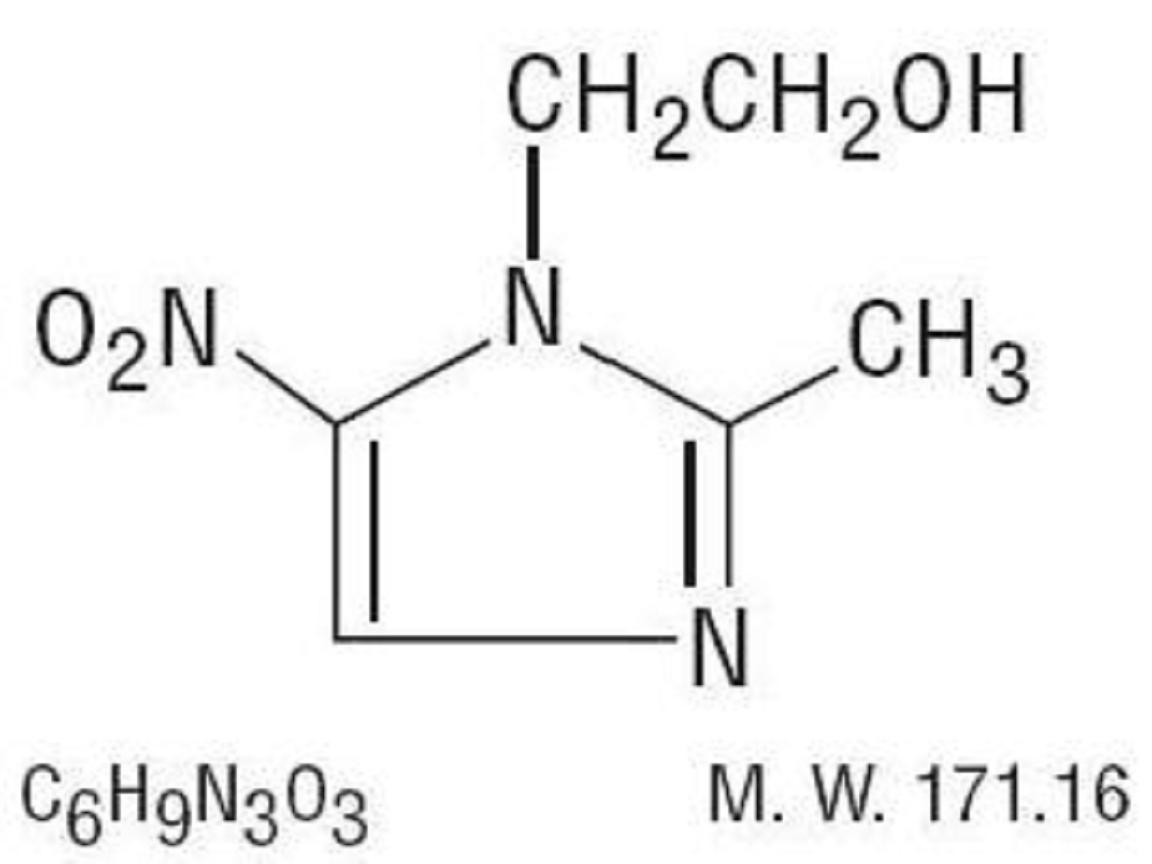
METRONIDAZOLE DESCRIPTION
CLINICAL PHARMACOLOGY
Microbiology
Susceptibility Tests
INDICATIONS & USAGE
Symptomatic TrichomoniasisAsymptomatic Trichomoniasis
Treatment of Asymptomatic Consorts
Amebiasis
Anaerobic Bacterial Infections
METRONIDAZOLE CONTRAINDICATIONS
WARNINGS
WARNINGS
Convulsive Seizures and Peripheral NeuropathyPRECAUTIONS
GeneralINFORMATION FOR PATIENTS
Drug InteractionsLABORATORY TESTS
DRUG INTERACTIONS
DRUG & OR LABORATORY TEST INTERACTIONS
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic EffectsPregnancy category B
NURSING MOTHERS
GERIATRIC USE
PEDIATRIC USE
METRONIDAZOLE ADVERSE REACTIONS
OVERDOSAGE
Treatment
DOSAGE & ADMINISTRATION
Trichomoniasis
In the Female
CONTRAINDICATIONS.)PRECAUTIONS, Pregnancy).
In the Male
Amebiasis
Adults
Pediatric Patients
Anaerobic Bacterial Infections
HOW SUPPLIED
STORAGE AND HANDLING
REFERENCES
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

MetronidazoleMetronidazole TABLET
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!