Metoprolol Tartrate and Hydrochlorothiazide
Sun Pharmaceutical Industries Limited
Metoprolol Tartrate and Hydrochlorothiazide Tablets, USPBeta-Blocker/Diuretic Antihypertensive
FULL PRESCRIBING INFORMATION: CONTENTS*
- METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
- METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
- OVERDOSAGE
- METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL-Label -50 mg/25 mg
- PRINCIPAL DISPLAY PANEL-Label -100 mg/25 mg
- PRINCIPAL DISPLAY PANEL-Label -100 mg/50 mg
FULL PRESCRIBING INFORMATION
Ischemic Heart Disease: Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have been reported. Even in the absence of overt angina pectoris, when discontinuing therapy, metoprolol should not be withdrawn abruptly, and patients should be cautioned against interruption of therapy without the physician’s advice (see PRECAUTIONS, Information for Patients).
METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE DESCRIPTION
1p

H
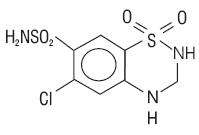
Inactive Ingredients.
CLINICAL PHARMACOLOGY
Metoprolol
In vitroin vivo12
121211
Pharmacokinetics
Plasma levels achieved are highly variable after oral administration. Only a small fraction of the drug (about 12%) is bound to human serum albumin. Metoprolol is a racemic mixture of R- and S-enantiomers. Less than 5% of an oral dose of metoprolol is recovered unchanged in the urine; the rest is excreted by the kidneys as metabolites that appear to have no clinical significance. The systemic availability and half-life of metoprolol in patients with renal failure do not differ to a clinically significant degree from those in normal subjects. Consequently, no reduction in dosage is usually needed in patients with chronic renal failure.
In elderly subjects with clinically normal renal function, there are no significant differences in metoprolol pharmacokinetics compared to young subjects.
Metoprolol is extensively metabolized by the cytochrome P450 enzyme system in the liver. The oxidative metabolism of metoprolol is under genetic control with a major contribution of the polymorphic cytochrome P450 isoform 2D6 (CYP2D6). There are marked ethnic differences in the prevalence of the poor metabolizers (PM) phenotype. Approximately 7% of Caucasians and less than 1% Asians are poor metabolizers.
Pharmacodynamics
Hydrochlorothiazide
Pharmacokinetics
Pharmacodynamics
METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE INDICATIONS AND USAGE
Metoprolol tartrate and hydrochlorothiazide tablets are indicated for the management of hypertension.
This fixed-combination drug is not indicated for initial therapy of hypertension. If the fixed combination represents the dose titrated to the individual patient’s needs, therapy with the fixed combination may be more convenient than with the separate components.METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE CONTRAINDICATIONS
Metoprolol
Metoprolol tartrate tablets are contraindicated in sinus bradycardia, heart block greater than first degree, cardiogenic shock, and overt cardiac failure (see WARNINGS).
Hypersensitivity to metoprolol and related derivatives, or to any of the excipients; hypersensitivity to other beta-blockers (cross sensitivity between beta-blockers can occur).
Sick-sinus syndrome.
Hydrochlorothiazide
WARNINGS
WARNINGS
Metoprolol
Cardiac Failure:
In Patients Without a History of Cardiac Failure:
Bronchospastic Diseases: PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol tartrate and hydrochlorothiazide tablets. Because of its relative beta1 selectivity, however, metoprolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta1 selectivity is not absolute, a beta2-stimulating agent should be administered concomitantly, and the lowest possible dose of metoprolol should be used. In these circumstances it would be prudent initially to administer metoprolol in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval (see DOSAGE AND ADMINISTRATION).
Major Surgery:
Diabetes and Hypoglycemia:
Pheochromocytoma:
Thyrotoxicosis:
Hydrochlorothiazide
Thiazides should be used with caution in patients with severe renal disease. In patients with renal disease, thiazides may precipitate azotemia. Cumulative effects of the drug may develop in patients with impaired renal function.
Thiazides should be used with caution in patients with impaired hepatic function or progressive liver disease, since minor alterations of fluid and electrolyte imbalance may precipitate hepatic coma.
Thiazides may add to or potentiate the action of other antihypertensive drugs. Potentiation occurs with ganglionic or peripheral adrenergic blocking drugs.
Sensitivity reactions are more likely to occur in patients with a history of allergy or bronchial asthma.
Acute Myopia and Secondary Angle-Closure Glaucoma:
PRECAUTIONS
General
Metoprolol: Metoprolol should be used with caution in patients with impaired hepatic function.
Hydrochlorothiazide: Laboratory TestsDrug/Drug Interactions
Drug/Drug Interactions
Information for Patients
Patients should be advised to take metoprolol tartrate and hydrochlorothiazide tablets regularly and continuously, as directed, with or immediately following meals. If a dose should be missed, the patient should take only the next scheduled dose (without doubling it). Patients should not discontinue metoprolol tartrate and hydrochlorothiazide tablets without consulting the physician.
Laboratory Tests
Metoprolol:
Hydrochlorothiazide:
Drug/Drug Interactions
Metoprolol: Catecholamine-depleting drugs (e.g., reserpine) may have an additive effect when given with beta-blocking agents. Patients treated with metoprolol plus a catecholamine depletor should therefore be closely observed for evidence of hypotension or marked bradycardia, which may produce vertigo, syncope, or postural hypotension.
Risk of Anaphylactic Reaction:
General Anesthetics
Some inhalation anesthetics may enhance the cardiodepressant effect of beta-blockers (see WARNINGS; Metoprolol; Major Surgery).
CYP2D6 Inhibitors
Potent inhibitors of the CYP2D6 enzyme may increase the plasma concentration of metoprolol. Strong inhibition of CYP2D6 would mimic the pharmacokinetics of CYP2D6 poor metabolizer. Caution should therefore be exercised when administering potent CYP2D6 inhibitors with metoprolol. Known clinically significant potent inhibitors of CYP2D6 are antidepressants such as fluoxetine, paroxetine or bupropion, antipsychotics such as thioridazine, antiarrhythmics such as quinidine or propafenone, antiretrovirals such as ritonavir, antihistamines such as diphenhydramine, antimalarials such as hydroxychloroquine or quinidine, antifungals such as terbinafine and medications for stomach ulcers such as cimetidine.
Clonidine
Hydrochlorothiazide:
Hypokalemia may develop during concomitant use of steroids or ACTH.
Insulin requirements in diabetic patients may be increased, decreased, or unchanged.
Thiazides may decrease arterial responsiveness to norepinephrine, but not enough to preclude effectiveness of the pressor agent for therapeutic use.
Thiazides may increase the responsiveness to tubocurarine.
Lithium renal clearance is reduced by thiazides, increasing the risk of lithium toxicity.
There have been rare reports in the literature of hemolytic anemia occurring with the concomitant use of hydrochlorothiazide and methyldopa.
Concurrent administration of some nonsteroidal anti-inflammatory agents may reduce the diuretic, natriuretic and antihypertensive effects of thiazide diuretics.
Cholestyramine and colestipol resins:
Drug/Laboratory Test Interactions
Hydrochlorothiazide: General, Hydrochlorothiazide, Calcium excretion
Carcinogenesis, Mutagenesis, Impairment of Fertility
Metoprolol Tartrate and Hydrochlorothiazide: Carcinogenicity and mutagenicity studies have not been conducted with metoprolol tartrate and hydrochlorothiazide tablets. Metoprolol tartrate and hydrochlorothiazide tablets produced no evidence of impaired fertility in male or female rats administered gavaged doses up to 200/50 mg/kg (100/50 times the maximum recommended daily human dose) prior to mating and throughout gestation and rearing of young.
Metoprolol: Long-term studies in animals have been conducted to evaluate carcinogenic potential. In a 2-year study in rats at three oral dosage levels of up to 800 mg/kg per day, there was no increase in the development of spontaneously occurring benign or malignant neoplasms of any type. The only histologic changes that appeared to be drug related were an increased incidence of generally mild focal accumulation of foamy macrophages in pulmonary alveoli and a slight increase in biliary hyperplasia. In a 21-month study in Swiss albino mice at three oral dosage levels of up to 750 mg/kg per day, benign lung tumors (small adenomas) occurred more frequently in female mice receiving the highest dose than in untreated control animals. There was no increase in malignant or total (benign plus malignant) lung tumors, or in the overall incidence of tumors or malignant tumors. This 21-month study was repeated in CD-1 mice, and no statistically or biologically significant differences were observed between treated and control mice of either sex for any type of tumor.
All mutagenicity tests performed (a dominant lethal study in mice, chromosome studies in somatic cells, a Salmonella/mammalian-microsome mutagenicity test, and a nucleus anomaly test in somatic interphase nuclei) were negative.
Hydrochlorothiazide:
Hydrochlorothiazide was not genotoxic in in vitro assays using strains TA 98, TA 100, TA 1535, TA 1537, and TA 1538 of Salmonella typhimurium (Ames assay) and in the Chinese Hamster Ovary (CHO) test for chromosomal aberrations, or in in vivo assays using mouse germinal cell chromosomes, Chinese hamster bone marrow chromosomes, and the Drosophila sex-linked recessive lethal trait gene. Positive test results were obtained only in the in vitro CHO Sister Chromatid Exchange (clastogenicity) and in the Mouse Lymphoma Cell (mutagenicity) assays, using concentrations of hydrochlorothiazide from 43 to 1300 mcg/mL, and in the Aspergillus nidulans nondisjunction assay at an unspecified concentration.
Pregnancy
Teratogenic Effects. Pregnancy Category C
Metoprolol Tartrate and Hydrochlorothiazide:
Metoprolol: Metoprolol has been shown to increase postimplantation loss and decrease neonatal survival in rats at doses up to 55.5 times the maximum daily human dose of 450 mg. Distribution studies in mice confirm exposure of the fetus when metoprolol is administered to the pregnant animal. These studies have revealed no evidence of teratogenicity.
Hydrochlorothiazide:
Nonteratogenic Effects
Hydrochlorothiazide:Nursing Mothers
Pediatric Use
Geriatric Use
WARNINGS
METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE ADVERSE REACTIONS
Metoprolol Tartrate and Hydrochlorothiazide
The following adverse reactions were reported in controlled clinical studies of the combination of metoprolol and hydrochlorothiazide.
Body as a Whole: Fatigue or lethargy and flu syndrome have each been reported in about 10 in 100 patients.
Nervous System: Dizziness or vertigo, drowsiness or somnolence, and headache have each occurred in about 10 in 100 patients. Nightmare has occurred in 1 in 100 patients.
Cardiovascular: Bradycardia has occurred in about 6 in 100 patients. Decreased exercise tolerance and dyspnea have each occurred in about 1 of 100 patients.
Digestive: Diarrhea, digestive disorder, dry mouth, nausea or vomiting, and constipation have each occurred in about 1 in 100 patients.
Metabolic and Nutritional: Hypokalemia has occurred in fewer than 10 in 100 patients. Edema, gout, and anorexia have each occurred in 1 in 100 patients.
Special Senses: Blurred vision, tinnitus, and earache have each been reported in 1 in 100 patients.
Skin: Sweating and purpura have each occurred in 1 in 100 patients.
Urogenital: Impotence has occurred in 1 in 100 patients.
Musculoskeletal: Muscle pain has occurred in 1 in 100 patients.
Metoprolol
Most adverse effects have been mild and transient.
Central Nervous System: Tiredness and dizziness have occurred in about 10 of 100 patients. Depression has been reported in about 5 of 100 patients. Mental confusion and short-term memory loss have been reported. Headache, nightmares, and insomnia have also been reported, but a drug relationship is not clear.
Cardiovascular: Shortness of breath and bradycardia have occurred in approximately 3 of 100 patients. Cold extremities; arterial insufficiency, usually of the Raynaud type; palpitations; and congestive heart failure have been reported. Gangrene in patients with preexisting severe peripheral circulatory disorders has also been reported very rarely (see CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS).
Respiratory: Wheezing (bronchospasm) has been reported in fewer than 1 of 100 patients (see WARNINGS). Rhinitis has also been reported.
Gastrointestinal: Diarrhea has occurred in about 5 of 100 patients. Nausea, gastric pain, constipation, flatulence, and heartburn have been reported in 1 of 100, or fewer, patients. Vomiting was a common occurrence. Postmarketing experience reveals very rare reports of hepatitis, jaundice and non-specific hepatic dysfunction. Isolated cases of transaminase, alkaline phosphatase, and lactic dehydrogenase elevations have also been reported.
Hypersensitive Reactions:
Miscellaneous:
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with metoprolol.
Potential Adverse Reactions
A variety of adverse reactions not listed above have been reported with other beta-adrenergic blocking agents and should be considered potential adverse reactions to metoprolol.
Central Nervous System: Reversible mental depression progressing to catatonia; visual disturbances; hallucinations; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block (see CONTRAINDICATIONS).
Hematologic: Agranulocytosis, nonthrombocytopenic purpura, thrombocytopenic purpura.
Hypersensitive Reactions:
Postmarketing Experience
The following adverse reactions have been reported during postapproval use of metoprolol: confusional state, an increase in blood triglycerides and a decrease in High Density Lipoprotein (HDL). Because these reports are from a population of uncertain size and are subject to confounding factors, it is not possible to reliably estimate their frequency.
Hydrochlorothiazide
The following adverse reactions have been observed, but there has not been enough systematic collection of data to support an estimate of their frequency. Consequently the reactions are categorized by organ systems and are listed in decreasing order of severity and not frequency.
Digestive: Pancreatitis, jaundice (intrahepatic cholestatic), sialadenitis, vomiting, diarrhea, cramping, nausea, gastric irritation, constipation, anorexia.
Cardiovascular: Orthostatic hypotension (may be potentiated by alcohol, barbiturates, or narcotics).
Neurologic: Vertigo, dizziness, transient blurred vision, headache, paresthesia, xanthopsia, weakness, restlessness.
Musculoskeletal: Muscle spasm.
Hematologic: Aplastic anemia, agranulocytosis, leukopenia, thrombocytopenia.
Metabolic: Hyperglycemia, glycosuria, hyperuricemia.
Hypersensitive Reactions: Necrotizing angiitis, Stevens-Johnson syndrome, respiratory distress including pneumonitis and pulmonary edema, purpura, urticaria, rash, photosensitivity.
OVERDOSAGE
Acute Toxicity
Several cases of overdosage with metoprolol have been reported, some leading to death. No deaths have been reported with hydrochlorothiazide.
Oral LD50’s (mg/kg): mice, 1158 (metoprolol); rats, 3090 (metoprolol), 2750 (hydrochlorothiazide).
Signs and Symptoms
Metoprolol: Potential signs and symptoms associated with overdosage with metoprolol are bradycardia, hypotension, bronchospasm, and cardiac failure.
Hydrochlorothiazide: The most prominent feature of poisoning is acute loss of fluid and electrolytes.
Cardiovascular: Tachycardia, hypotension, shock.
Neuromuscular: Weakness, confusion, dizziness, cramps of the calf muscles, paresthesia, fatigue, impairment of consciousness.
Digestive: Nausea, vomiting, thirst.
Renal: Polyuria, oliguria, or anuria (due to hemoconcentration).
Laboratory Findings: Hypokalemia, hyponatremia, hypochloremia, alkalosis; increased BUN (especially in patients with renal insufficiency).
Combined Poisoning: Signs and symptoms may be aggravated or modified by concomitant intake of antihypertensive medication, barbiturates, curare, digitalis (hypokalemia), corticosteroids, narcotics, or alcohol.
Treatment
There is no specific antidote.
On the basis of the pharmacologic actions of metoprolol and hydrochlorothiazide, the following general measures should be employed:
Elimination of the Drug: Inducement of vomiting, gastric lavage, and activated charcoal.
Bradycardia: Atropine should be administered. If there is no response to vagal blockade, isoproterenol should be administered cautiously.
Hypotension: The patient’s legs should be elevated, and lost fluid and electrolytes (potassium, sodium) should be replaced. A vasopressor should be administered, e.g., norepinephrine or dopamine.
Bronchospasm: A beta2-stimulating agent and/or a theophylline derivative should be administered.
Cardiac Failure: A digitalis glycoside and diuretic should be administered. In shock resulting from inadequate cardiac contractility, administration of dobutamine, isoproterenol, or glucagon may be considered.
Surveillance:METOPROLOL TARTRATE AND HYDROCHLOROTHIAZIDE DOSAGE AND ADMINISTRATION
Dosage should be determined by individual titration (see INDICATIONS AND USAGE).
Hydrochlorothiazide is usually given at a dosage of 12.5 to 50 mg per day. The usual initial dosage of metoprolol tartrate tablets is 100 mg daily in single or divided doses. Dosage may be increased gradually until optimum blood pressure control is achieved. The effective dosage range is 100 to 450 mg per day. While once-daily dosing is effective and can maintain a reduction in blood pressure throughout the day, lower doses (especially 100 mg) may not maintain a full effect at the end of the 24-hour period, and larger or more frequent daily doses may be required. This can be evaluated by measuring blood pressure near the end of the dosing interval to determine whether satisfactory control is being maintained throughout the day. Beta1 selectivity diminishes as dosage of metoprolol tartrate tablets is increased.
|
Metoprolol Tartrate
and Hydrochlorothiazide |
Dosage
|
| Tablets of 50 mg/25 mg |
2 tablets per day in single or divided doses |
| Tablets of 100 mg/25 mg |
1 to 2 tablets per day in single or divided doses |
| Tablets of 100 mg/50 mg |
1 tablet per day in single or divided doses |
HOW SUPPLIED
Tablets 50 mg/25 mg
Capsule-shaped, yellow, debossed with ‘S’ and ‘370’ on either side of breakline on one side and plain on other side, 50 mg of metoprolol tartrate and 25 mg of hydrochlorothiazide
Bottles of 30’s with Child Resistant Cap……………NDC 62756-370-83
Bottles of 100’s with Child Resistant Cap…………..NDC 62756-370-88
Bottles of 100’s with Non Child Resistant Cap……..NDC 62756-370-08
Bottles of 1000’s with Non Child Resistant Cap…....NDC 62756-370-18
Tablets 100 mg/25 mg
Capsule-shaped, pink, debossed with ‘S’ and ‘368’ on either side of breakline on one side and plain on other side, 100 mg of metoprolol tartrate and 25 mg of hydrochlorothiazide
Bottles of 30’s with Child Resistant Cap……………NDC 62756-368-83
Bottles of 100’s with Child Resistant Cap…………..NDC 62756-368-88
Bottles of 100’s with Non Child Resistant Cap……..NDC 62756-368-08
Bottles of 1000’s with Non Child Resistant Cap…....NDC 62756-368-18
Tablets 100 mg/50 mg
Capsule-shaped, yellow, debossed with ‘S’ and ‘369’ on either side of breakline on one side and plain on other side, 100 mg of metoprolol tartrate and 50 mg of hydrochlorothiazide
Bottles of 30’s with Child Resistant Cap……………NDC 62756-369-83
Bottles of 100’s with Child Resistant Cap…………..NDC 62756-369-88
Bottles of 100’s with Non Child Resistant Cap……..NDC 62756-369-08
Bottles of 1000’s with Non Child Resistant Cap…....NDC 62756-369-18
Store at 20° to 25°C (68° to 77°F); excursions permitted between 15° and 30°C (59° and 86°F) [see USP Controlled Room Temperature]. Protect from moisture.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
To report SUSPECTED ADVERSE REACTIONS, contact CARACO Pharmaceutical Laboratories Ltd. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Caraco Pharmaceutical Laboratories, Ltd.
Sun Pharmaceutical Ind. Ltd.
PRINCIPAL DISPLAY PANEL-Label -50 mg/25 mg
NDC 62756-370-88
Metoprolol Tartrate and Hydrochlorothiazide Tablets, USP
50 mg/25 mg
Rx only
100 TABLETS
SUN PHARMA

PRINCIPAL DISPLAY PANEL-Label -100 mg/25 mg
NDC 62756-368-88
Metoprolol Tartrate and Hydrochlorothiazide Tablets, USP
100 mg/25 mg
Rx only
100 TABLETS
SUN PHARMA

PRINCIPAL DISPLAY PANEL-Label -100 mg/50 mg
NDC 62756-369-88
Metoprolol Tartrate and Hydrochlorothiazide Tablets, USP
100 mg/50 mg
Rx only
100 TABLETS
SUN PHARMA

Metoprolol Tartrate and HydrochlorothiazideMetoprolol Tartrate and Hydrochlorothiazide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metoprolol Tartrate and HydrochlorothiazideMetoprolol Tartrate and Hydrochlorothiazide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metoprolol Tartrate and HydrochlorothiazideMetoprolol Tartrate and Hydrochlorothiazide TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||