Metoprolol Tartrate
FULL PRESCRIBING INFORMATION: CONTENTS*
- METOPROLOL TARTRATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- METOPROLOL TARTRATE INDICATIONS AND USAGE
- METOPROLOL TARTRATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- METOPROLOL TARTRATE ADVERSE REACTIONS
- OVERDOSAGE
- METOPROLOL TARTRATE DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- PRINCIPAL DISPLAY PANEL
FULL PRESCRIBING INFORMATION
|
Ischemic Heart Disease |
|
|
|
Bronchospastic Diseases |
|
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol. Because of its relative beta1 selectivity, however, metoprolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta1 selectivity is not absolute, a beta2-stimulating agent should be administered concomitantly, and the lowest possible dose of metoprolol tartrate should be used. In these circumstances it would be prudent initially to administer metoprolol in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval. (See DOSAGE AND ADMINISTRATION.) |
|
|
|
Major Surgery |
|
The necessity or desirability of withdrawing beta-blocking
therapy, including metoprolol, prior to major surgery is
controversial; the impaired ability of the heart to respond to
reflex adrenergic stimuli may augment the risks of general
anesthesia and surgical procedures. |
|
|
METOPROLOL TARTRATE DESCRIPTION
Metoprolol tartrate, USP is a selective beta1-adrenoreceptor blocking agent, available as 25, 50 and 100 mg tablets for oral administration. Metoprolol tartrate is (±)-1-(isopropylamino)-3-[p-(2-methoxyethyl)phenoxy]-2-propanol (2:1) dextro-tartrate salt. Its structural formula is:
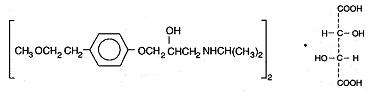
Metoprolol tartrate is a white, practically odorless, crystalline powder with a
molecular weight of 684.82. It is very soluble in water; freely soluble in
methylene chloride, in chloroform, and in alcohol; slightly soluble in acetone;
and insoluble in ether.
Each tablet for oral administration contains 25
mg, 50 mg or 100 mg of metoprolol tartrate.
The tablets contain the
following inactive ingredients: microcrystalline cellulose, corn starch, sodium
starch glycollate, colloidal silicon dioxide, sodium lauryl sulfate, talc,
magnesium stearate, hypromellose, titanium dioxide, polyethylene glycol and
polysorbate 80. In addition, 50 mg tablet contains D&C Red #30 Aluminium
Lake and 100 mg tablet contains FD&C Blue #2 Aluminium Lake as coloring
agents.
CLINICAL PHARMACOLOGY
Metoprolol tartrate is a beta-adrenergic receptor blocking agent. In vitro and in vivo animal
studies have shown that it has a preferential effect on beta1 adrenoreceptors, chiefly located in cardiac muscle. This
preferential effect is not absolute, however, and at higher doses, metoprolol
also inhibits beta2 adrenoreceptors, chiefly located in
the bronchial and vascular musculature.
Clinical pharmacology studies
have confirmed the beta-blocking activity of metoprolol in man, as shown by (1)
reduction in heart rate and cardiac output at rest and upon exercise, (2)
reduction of systolic blood pressure upon exercise, (3) inhibition of
isoproterenol-induced tachycardia, and (4) reduction of reflex orthostatic
tachycardia.
Relative beta1 selectivity has been
confirmed by the following: (1) In normal subjects, metoprolol is unable to
reverse the beta2-mediated vasodilating effects of
epinephrine. This contrasts with the effect of
nonselective (beta1 plus beta2)
beta-blockers, which completely reverse the vasodilating effects of epinephrine.
(2) In asthmatic patients, metoprolol reduces FEV1 and FVC significantly less than a nonselective beta-blocker,
propranolol, at equivalent beta1-receptor blocking
doses.
Metoprolol has no intrinsic sympathomimetic activity, and
membrane-stabilizing activity is detectable only at doses much greater than
required for beta-blockade. Metoprolol crosses the blood-brain barrier and has
been reported in the CSF in a concentration 78% of the simultaneous plasma
concentration. Animal and human experiments indicate that metoprolol slows the
sinus rate and decreases AV nodal conduction.
In controlled clinical
studies, metoprolol tartrate has been shown to be an effective antihypertensive
agent when used alone or as concomitant therapy with thiazide-type diuretics, at
dosages of 100 to 450 mg daily. In controlled, comparative, clinical studies,
metoprolol has been shown to be as effective an antihypertensive agent as
propranolol, methyldopa, and thiazide-type diuretics, and to be equally
effective in supine and standing positions.
The mechanism of the
antihypertensive effects of beta-blocking agents has not been
elucidated. However, several possible mechanisms have been proposed: (1)
competitive antagonism of catecholamines at peripheral (especially cardiac)
adrenergic neuron sites, leading to decreased cardiac output; (2) a central
effect leading to reduced sympathetic outflow to the periphery; and (3)
suppression of renin activity.
By blocking catecholamine-induced
increases in heart rate, in velocity and extent of myocardial contraction, and
in blood pressure, metoprolol reduces the oxygen requirements of the heart at
any given level of effort, thus making it useful in the long-term management of
angina pectoris. However, in patients with heart failure, beta-adrenergic
blockade may increase oxygen requirements by increasing left ventricular fiber
length and end-diastolic pressure.
Although beta-adrenergic receptor
blockade is useful in the treatment of angina and hypertension, there are
situations in which sympathetic stimulation is vital. In patients with severely
damaged hearts, adequate ventricular function may depend on sympathetic drive.
In the presence of AV block, beta-blockade may prevent the necessary
facilitating effect of sympathetic activity on conduction. Beta2-adrenergic blockade results in passive bronchial constriction
by interfering with endogenous adrenergic bronchodilator activity in patients
subject to bronchospasm and may also interfere with exogenous bronchodilators in
such patients.
In controlled clinical trials, metoprolol tartrate,
administered two or four times daily, has been shown to be an effective
antianginal agent, reducing the number of angina attacks and increasing exercise
tolerance. The dosage used in these studies ranged from 100 to 400 mg daily. A
controlled, comparative, clinical trial showed that metoprolol was
indistinguishable from propranolol in the treatment of angina
pectoris.
In a large (1,395 patients randomized), double-blind,
placebo-controlled clinical study, metoprolol was shown to reduce 3-month
mortality by 36% in patients with suspected or definite myocardial
infarction.
Patients were randomized and treated as soon as possible
after their arrival in the hospital, once their clinical condition had
stabilized and their hemodynamic status had been carefully evaluated. Subjects
were ineligible if they had hypotension, bradycardia, peripheral signs of shock,
and/or more than minimal basal rales as signs of congestive heart failure.
Initial treatment consisted of intravenous followed by oral administration of
metoprolol tartrate or placebo, given in a coronary care or comparable unit.
Oral maintenance therapy with metoprolol or placebo was then continued for 3
months. After this double-blind period, all patients were given metoprolol and
followed up to 1 year.
The median delay from the onset of symptoms to the
initiation of therapy was 8 hours in both the metoprolol and placebo treatment
groups. Among patients treated with metoprolol, there were comparable reductions
in 3-month mortality for those treated early (≤ 8 hours) and those in whom
treatment was started later. Significant reductions in the incidence of
ventricular fibrillation and in chest pain following initial intravenous therapy
were also observed with metoprolol and were independent of the interval, between
onset of symptoms and initiation of therapy.
The precise mechanism of
action of metoprolol in patients with suspected or definite myocardial
infarction is not known.
In this study, patients treated with metoprolol
received the drug both very early (intravenously) and during a subsequent
3-month period, while placebo patients received no beta-blocker treatment for
this period. The study thus was able to show a benefit from the overall
metoprolol regimen but cannot separate the benefit of very early intravenous
treatment from the benefit of later beta-blocker therapy. Nonetheless, because
the overall regimen showed a clear beneficial effect on survival without
evidence of an early adverse effect on survival, one acceptable dosage regimen
is the precise regimen used in the trial. Because the specific benefit of very
early treatment remains to be defined however, it is also reasonable to
administer the drug orally to patients at a later time as is recommended for
certain other beta-blockers.
METOPROLOL TARTRATE INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATIONCONTRAINDICATIONSWARNINGSDOSAGE AND ADMINISTRATION
METOPROLOL TARTRATE CONTRAINDICATIONS
WARNINGS
WARNINGS
WARNINGS
WARNINGS
Following abrupt cessation of therapy with certain beta-blocking agents, exacerbations of angina pectoris and, in some cases, myocardial infarction have occurred. When discontinuing chronically administered metoprolol, particularly in patients with ischemic heart disease, the dosage should be gradually reduced over a period of 1 to 2 weeks and the patient should be carefully monitored. If angina markedly worsens or acute coronary insufficiency develops, metoprolol administration should be reinstated promptly, at least temporarily, and other measures appropriate for the management of unstable angina should be taken. Patients should be warned against interruption or discontinuation of therapy without the physician's advice. Because coronary artery disease is common and may be unrecognized, it may be prudent not to discontinue metoprolol therapy abruptly even in patients treated only for hypertension.
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol. Because of its relative beta1 selectivity, however, metoprolol may be used with caution in patients with bronchospastic disease who do not respond to, or cannot tolerate, other antihypertensive treatment. Since beta1 selectivity is not absolute, a beta2-stimulating agent should be administered concomitantly, and the lowest possible dose of metoprolol tartrate should be used. In these circumstances it would be prudent initially to administer metoprolol in smaller doses three times daily, instead of larger doses two times daily, to avoid the higher plasma levels associated with the longer dosing interval. (See DOSAGE AND ADMINISTRATION.)
The necessity or desirability of withdrawing beta-blocking therapy, including metoprolol, prior to major surgery is controversial; the impaired ability of the heart to respond to reflex adrenergic stimuli may augment the risks of general anesthesia and surgical procedures.
Metoprolol, like other beta-blockers, is a competitive inhibitor of beta-receptor agonists, and its effects can be reversed by administration of such agents, e.g., dobutamine or isoproterenol. However, such patients may be subject to protracted severe hypotension. Difficulty in restarting and maintaining the heartbeat has also been reported with beta-blockers.
Metoprolol should be used with caution in diabetic patients if a beta-blocking agent is required. Beta-blockers may mask tachycardia occurring with hypoglycemia, but other manifestations such as dizziness and sweating may not be significantly affected.
In patients known to have, or suspected of having, a pheochromocytoma, metoprolol is contraindicated (see CONTRAINDIATIONS). If metoprolol is required, it should be given in combination with an alpha blocker, and only after the alpha blocker has been initiated. Administration of beta-blockers alone in the setting of pheochromocytoma have been associated with a paradoxical increase in blood pressure due to the attenuation of beta-mediated vasodilatation in skeletal muscle.
Beta-adrenergic blockade may mask certain clinical signs (e.g., tachycardia) of hyperthyroidism. Patients suspected of developing thyrotoxicosis should be managed carefully to avoid abrupt withdrawal of beta-blockade, which might precipitate a thyroid storm.
PATIENTS WITH BRONCHOSPASTIC DISEASES SHOULD, IN GENERAL, NOT RECEIVE BETA-BLOCKERS, including metoprolol. Because of its relative beta1 selectivity, metoprolol may be used with extreme caution in patients with bronchospastic disease. Because it is unknown to what extent beta2-stimulating agents may exacerbate myocardial ischemia and the extent of infarction, these agents should not be used prophylactically. If bronchospasm not related to congestive heart failure occurs, metoprolol should be discontinued. A theophylline derivative or a beta2 agonist may be administered cautiously, depending on the clinical condition of the patient. Both theophylline derivatives and beta2 agonists may produce serious cardiac arrhythmias.
PRECAUTIONS
WARNINGS, Major Surgery
METOPROLOL TARTRATE ADVERSE REACTIONS
Central Nervous System:
Cardiovascular: CONTRAINDICATIONSWARNINGSPRECAUTIONS
Respiratory: WARNINGS
Gastrointestinal:
Hypersensitive Reactions:
Miscellaneous:
Central Nervous System:
Cardiovascular: CLINICAL PHARMACOLOGY
| |
Metoprolol
|
Placebo
|
| Hypotension (systolic BP less than 90 mm Hg) |
27.4% |
23.2% |
| Bradycardia (heart rate less than 40
beats/min) |
15.9% |
6.7% |
| Second- or third-degree heart block |
4.7% |
4.7% |
| First-degree heart block (P-R greater than or equal to 0.26
sec) |
5.3% |
1.9% |
| Heart failure |
27.5% |
29.6% |
Respiratory:
Gastrointestinal:
Dermatologic:
Miscellaneous:
Central Nervous System:
Cardiovascular: CONTRAINDICATIONS
Hematologic:
Hypersensitive Reactions:
Postmarketing Experience
OVERDOSAGE
Acute Toxicity: Several cases of overdosage have been
reported, some leading to death.
Oral LD50’s
(mg/kg): mice, 1158 to 2460; rats, 3090 to 4670.
WARNINGS: Myocardial Infarction
Elimination of the Drug:
Bradycardia:
Hypotension:
Bronchospasm: 2
Cardiac Failure:
METOPROLOL TARTRATE DOSAGE AND ADMINISTRATION
1
WARNINGS
Late Treatment
WARNINGS
HOW SUPPLIED
Metoprolol Tartrate Tablets, USP are available as follows:
Tablets 25 mg
are white round shaped, film
coated tablets debossed with ‘C over 73’ on one side and deep break line on
other side.
| Bottles of 30 |
NDC 54868-5021-0 |
| Bottles of 60 |
NDC 54868-5021-1 |
| Bottles of 90 |
NDC 54868-5021-3 |
| Bottles of 100 |
NDC 54868-5021-2 |
| Bottles of 120 |
NDC 54868-5021-5 |
| Bottles of 180 |
NDC 54868-5021-4 |
Tablets 50
mg
are pink round shaped, film coated tablets debossed with ‘C
over 74’ on one side and deep break line on other
side.
| Bottles of 10 |
NDC 54868-2989-4 |
| Bottles of 15 |
NDC 54868-2989-6 |
| Bottles of 30 |
NDC 54868-2989-2 |
| Bottles of 60 |
NDC 54868-2989-3 |
| Bottles of 90 |
NDC 54868-2989-5 |
| Bottles of 100 |
NDC 54868-2989-0 |
| Bottles of 180 |
NDC 54868-2989-7 |
Tablets
100 mg
are light blue round shaped, film coated tablets debossed
with ‘C over 75’ on one side and deep break line on other
side.
| Bottles of 30 |
NDC 54868-2990-0 |
| Bottles of 45 |
NDC 54868-2990-4 |
| Bottles of 60 |
NDC 54868-2990-3 |
| Bottles of 100 |
NDC 54868-2990-2 |
| Bottles of 180 |
NDC 54868-2990-5 |
Store at 20° to 25°C (68° to
77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled
Room Temperature].
Protect from
moisture.
Dispense in a tight,
light-resistant container as defined in the USP using a child-resistant
closure.
Manufactured for:
Aurobindo Pharma USA,
Inc.
2400 Route 130 North
Dayton, NJ 08810
Manufactured
by:
Aurobindo Pharma Limited
Hyderabad-500 072,
India
Revised: 01/2009
Relabeling and Repackaging by:
Physicians Total Care, Inc.
Tulsa, OK 74146
PRINCIPAL DISPLAY PANEL
Metoprolol Tartrate Tablets, USP
25 mg

50 mg

100 mg

Metoprolol TartrateMetoprolol Tartrate TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metoprolol TartrateMetoprolol Tartrate TABLET, FILM COATED
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Metoprolol TartrateMetoprolol Tartrate TABLET, FILM COATED
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||