Metformin Hydrochloride
FULL PRESCRIBING INFORMATION: CONTENTS*
- Lactic Acidosis
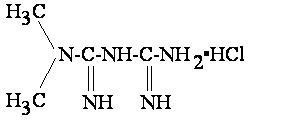
- METFORMIN HYDROCHLORIDE DESCRIPTION
- CLINICAL PHARMACOLOGY
- INDICATIONS & USAGE
- METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
- PRECAUTIONS
- INFORMATION FOR PATIENTS
- LABORATORY TESTS
- DRUG INTERACTIONS
- CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- PREGNANCY
- NURSING MOTHERS
- PEDIATRIC USE
- GERIATRIC USE
- METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
- PEDIATRIC USE
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- STORAGE AND HANDLING
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
Lactic Acidosis
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumlation during treatment with metformin; when it occurs, it is fatal in approximately 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by elevated blood lactate (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels ug/mL are generally found.The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/ 1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patientage. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see also PRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patientphysician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see also PRECAUTIONS). Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling (see also PRECAUTIONS).
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery (see also CONTRAINDICATIONSand PRECAUTIONS).
METFORMIN HYDROCHLORIDE DESCRIPTION
System Components and Performance:
CLINICAL PHARMACOLOGY
Mechanism of ActionPRECAUTIONS) and does not cause hyperinsulinemia. With metformin therapy, insulin secretion remains unchanged while fasting insulin levels and day-long plasma insulin response may actually decrease.
Pharmacokinetics
Absorption and Bioavailability
Distribution
Metabolism and Elimination
Special Populations
Patients with Type 2 Diabetes
Renal Insufficiency
In patients with decreased renal function (based on measured creatinine clearance), the plasma and blood half-life of metformin is prolonged and the renal clearance is decreased in proportion to the decrease in creatinine clearance (see Table 1; also seeWARNINGS).
Hepatic Insufficiency
Geriatrics
Table 1). Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced (seeWARNINGSandDOSAGE AND ADMINISTRATION).
Table 1Select Mean (Metformin Pharmacokinetic Parameters Following Single or Multiple Oral Doses of Metformin Hydrochloride Tablets
Pediatrics
Gender
Race
Clinical Studies
Metformin hydrochloride tablets
Table 2).
Table 2Metformin Hydrochloride Tablets vs Placebo Summary of Mean Changes from Baseline* in Fasting Plasma Glucose, HbAlc,and Body Weight, at Final Visit (29-week study)
Table 3Combined Metformin Hydrochloride Tablets/Glyburide (Comb) vs Glyburide (Glyb) or Metformin Hydrochloride Tablets (Met) monotherapy: Summary of Mean Changes from Baseline* in Fasting Plasma Glucose, HbA1c, and Body Weight, at Final Visit (29-week study)
Table 4).
Table 4Summary of Mean Percent Change From Baseline of Major Serum Lipid Variables at Final Visit (29-week studies)
Tables 2and3).
Table 5). Patients randomized to receive metformin hydrochloride tablets plus insulin achieved a reduction in HbA1c of 2.10%, compared to a 1.56% reduction in HbA1c achieved by insulin plus placebo. The improvement in glycemic control was achieved at the final study visit with 16% less insulin, 93.0 U/day vs 110.6 U/day, metformin hydrochloride tablets plus insulin versus insulin plus placebo, respectively, p=0.04.
Table 5Combined Metformin Hydrochloride Tablets/Insulin vs Placebo/Insulin Summary of Mean Changes from Baseline in HbA1c and Daily Insulin Dose
Metformin hydrochloride extended-release tablets
Table 6.
Table 6Summary of Mean Changes from Baseline* in HbAlc, Fasting Plasma Glucose, and Body Weight at Final Visit (16-week study)
DOSAGE AND ADMINISTRATIONfor dosing recommendations for metformin hydrochloride tablets and metformin hydrochloride extended-release tablets).
Table 7.
Table 7Summary of Mean Changes from Baseline *in HbA1c,Fasting PlasmaGlucose, and Body Weight at Week 12 and at Final visit (24-week study)
*
(-0.04, 0.31)
DOSAGE AND ADMINISTRATION).
Table 8.
Table 8Summary of Mean Percent Changes from Baseline *in Major Lipid Variables at Final Visit (16-week study)
*
Table 9.
Table 9Summary of Mean Percent Changes from Baseline *in MajorLipid Variables at Final Visit (24-week study)
*
Pediatric Clinical Studies
Table 10).
Table 10Metformin Hydrochloride Tablets Vs Placebo (Pediatricsa) Summary of Mean Changes from Baseline* in Plasma Glucose and Body Weight at Final Visit
INDICATIONS & USAGE
METFORMIN HYDROCHLORIDE CONTRAINDICATIONS
WARNINGSandPRECAUTIONS).
PRECAUTIONS).
Lactic Acidosis:
Lactic acidosis is a rare, but serious, metabolic complication that can occur due to metformin accumulation during treatment with metformin; when it occurs, it is fatal in approximatelty 50% of cases. Lactic acidosis may also occur in association with a number of pathophysiologic conditions, including diabetes mellitus, and whenever there is significant tissue hypoperfusion and hypoxemia. Lactic acidosis is characterized by ek\levated blood lactate levels (>5 mmol/L), decreased blood pH, electrolyte disturbances with an increased anion gap, and an increased lactate/pyruvate ratio. When metformin is implicated as the cause of lactic acidosis, metformin plasma levels >5 ug/mL are generally found.
The reported incidence of lactic acidosis in patients receiving metformin hydrochloride is very low (approximately 0.03 cases/1000 patient-years, with approximately 0.015 fatal cases/ 1000 patient-years). In more than 20,000 patient-years exposure to metformin in clinical trials, there were no reports of lactic acidosis. Reported cases have occurred primarily in diabetic patients with significant renal insufficiency, including both intrinsic renal disease and renal hypoperfusion, often in the setting of multiple concomitant medical/surgical problems and multiple concomitant medications. Patients with congestive heart failure requiring pharmacologic management, in particular those with unstable or acute congestive heart failure who are at risk of hypoperfusion and hypoxemia, are at increased risk of lactic acidosis. The risk of lactic acidosis increases with the degree of renal dysfunction and the patientage. The risk of lactic acidosis may, therefore, be significantly decreased by regular monitoring of renal function in patients taking metformin and by use of the minimum effective dose of metformin. In particular, treatment of the elderly should be accompanied by careful monitoring of renal function. Metformin treatment should not be initiated in patientsyears of age unless measurement of creatinine clearance demonstrates that renal function is not reduced, as these patients are more susceptible to developing lactic acidosis. In addition, metformin should be promptly withheld in the presence of any condition associated with hypoxemia, dehydration, or sepsis. Because impaired hepatic function may significantly limit the ability to clear lactate, metformin should generally be avoided in patients with clinical or laboratory evidence of hepatic disease. Patients should be cautioned against excessive alcohol intake, either acute or chronic, when taking metformin, since alcohol potentiates the effects of metformin hydrochloride on lactate metabolism. In addition, metformin should be temporarily discontinued prior to any intravascular radiocontrast study and for any surgical procedure (see also PRECAUTIONS).
The onset of lactic acidosis often is subtle, and accompanied only by nonspecific symptoms such as malaise, myalgias, respiratory distress, increasing somnolence, and nonspecific abdominal distress. There may be associated hypothermia, hypotension, and resistant bradyarrhythmias with more marked acidosis. The patient and the patientphysician must be aware of the possible importance of such symptoms and the patient should be instructed to notify the physician immediately if they occur (see also PRECAUTIONS). Metformin should be withdrawn until the situation is clarified. Serum electrolytes, ketones, blood glucose, and if indicated, blood pH, lactate levels, and even blood metformin levels may be useful. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Levels of fasting venous plasma lactate above the upper limit of normal but less than 5 mmol/L in patients taking metformin do not necessarily indicate impending lactic acidosis and may be explainable by other mechanisms, such as poorly controlled diabetes or obesity, vigorous physical activity, or technical problems in sample handling (see also PRECAUTIONS).
Lactic acidosis should be suspected in any diabetic patient with metabolic acidosis lacking evidence of ketoacidosis (ketonuria and ketonemia).
Lactic acidosis is a medical emergency that must be treated in a hospital setting. In a patient with lactic acidosis who is taking metformin, the drug should be discontinued immediately and general supportive measures promptly instituted. Because metformin hydrochloride is dialyzable (with a clearance of up to 170 mL/min under good hemodynamic conditions), prompt hemodialysis is recommended to correct the acidosis and remove the accumulated metformin. Such management often results in prompt reversal of symptoms and recovery (see also CONTRAINDICATIONSand PRECAUTIONS).
PRECAUTIONS
GeneralWARNINGSandDOSAGE AND ADMINISTRATION).
PRECAUTIONS:Drug Interactions), should be used with caution.
CONTRAINDICATIONS). Therefore, in patients in whom any such study is planned, metformin should be temporarily discontinued at the time of or prior to the procedure, and withheld for 48 hours subsequent to the procedure and reinstituted only after renal function has been re-evaluated and found to be normal.
PRECAUTIONS:Laboratory Tests).
WARNINGS).
INFORMATION FOR PATIENTS
WARNINGSandPRECAUTIONSsections, should be explained to patients. Patients should be advised to discontinue metformin immediately and to promptly notify their health practitioner if unexplained hyperventilation, myalgia, malaise, unusual somnolence, or other nonspecific symptoms occur. Once a patient is stabilized on any dose level of metformin, gastrointestinal symptoms, which are common during initiation of metformin therapy, are unlikely to be drug related. Later occurrence of gastrointestinal symptoms could be due to lactic acidosis or other serious disease.
Patient Informationprinted below).
LABORATORY TESTS
DOSAGE AND ADMINISTRATION).DRUG INTERACTIONS
(Clinical Evaluation of Drug Interactions Conducted with Metformin Hydrochloride Tablets)DOSAGE AND ADMINISTRATION:Concomitant Metformin Hydrochloride Tablets or Metformin Hydrochloride Extended-release Tablets and Oral Sulfonylurea Therapy in Adult Patients).
CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
PREGNANCY
Teratogenic Effects: Pregnancy Category BNURSING MOTHERS
PEDIATRIC USE
CLINICAL PHARMACOLOGY:Pediatric Clinical Studies). In this study, adverse effects were similar to those described in adults (seeADVERSE REACTIONS:Pediatric Patients). A maximum daily dose of 2000 mg is recommended (seeDOSAGE AND ADMINISTRATION:Recommended Dosing Schedule: Pediatrics).GERIATRIC USE
CONTRAINDICATIONS,WARNINGS, andCLINICAL PHARMACOLOGY:Pharmacokinetics). Because aging is associated with reduced renal function, metformin should be used with caution as age increases. Care should be taken in dose selection and should be based on careful and regular monitoring of renal function. Generally, elderly patients should not be titrated to the maximum dose of metformin (see alsoWARNINGSandDOSAGE AND ADMINISTRATION).METFORMIN HYDROCHLORIDE ADVERSE REACTIONS
Table 11.Table 11Most Common Adverse Reactions (>5.0 Percent) in a Placebo-Controlled Clinical Study of Metformin Hydrochloride Tablets Monotherapy *
*
Table 12.
Table 12Most Common Adverse Reactions (>5.0 Percent) in Placebo-ControlledStudies of Metformin Hydrochloride Extended-release Tablets *
*
PEDIATRIC USE
OVERDOSAGE
WARNINGS). Metformin is dialyzable with a clearance of up to 170 mL/min under good hemodynamic conditions. Therefore, hemodialysis may be useful for removal of accumulated drug from patients in whom metformin overdosage is suspected.DOSAGE & ADMINISTRATION
Recommended Dosing Schedule), fasting plasma glucose should be used to determine the therapeutic response to metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately three months.The therapeutic goal should be to decrease both fasting plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of metformin hydrochloride tablets or metformin hydrochloride extended-release tablets, either when used as monotherapy or in combination with sulfonylurea or insulin.
Metformin hydrochloride extended-release tablets must be swallowed whole and never crushed or chewed.Occasionally, the inactive ingredients of metformin hydrochloride extended-release tablets will be eliminated in the feces as a soft, hydrated mass (seePatient Informationprinted below).
Recommended Dosing Schedule
Adults -In general, clinically significant responses are not seen at doses below 1500 mg per day. However, a lower recommended starting dose and gradually increased dosage is advised to minimize gastrointestinal symptoms.
CLINICAL PHARMACOLOGY,Clinical Studies).
CLINICAL PHARMACOLOGY:Clinical Studies).
Pediatrics -The usual starting dose of metformin hydrochloride tablet is 500 mg twice a day, given with meals. Dosage increases should be made in increments of 500 mg weekly up to a maximum of 2000 mg per day, given in divided doses. Safety and effectiveness of metformin hydrochloride extended-release tablets in pediatric patients have not been established.
Transfer From Other Antidiabetic Therapy
Concomitant Metformin Hydrochloride Tablets or Metformin Hydrochloride Extended-release Tablets and Oral Sulfonylurea Therapy in Adult Patients
CLINICAL PHARMACOLOGY:Clinical Studies). However, attempts should be made to identify the minimum effective dose of each drug to achieve this goal. With concomitant metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and sulfonylurea therapy, the risk of hypoglycemia associated with sulfonylurea therapy continues and may be increased. Appropriate precautions should be taken (see Package Insert of the respective sulfonylurea).
Concomitant Metformin Hydrochloride Tablets or Metformin Hydrochloride Extended-release Tablets and Insulin Therapy in Adult Patients
Specific Patient Populations
WARNINGS).
HOW SUPPLIED
Metformin Hydrochloride Tablets, USPMetformin Hydrochloride Extended-release Tablets
STORAGE AND HANDLING
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATIONWhat are metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
WARNING: A small number of people who have taken metformin hydrochloride tablets have developed a serious condition called lactic acidosis. Lactic acidosis is caused by a buildup of lactic acid in the blood. This happens more often in people with kidney problems. Most people with kidney problems should not take metformin hydrochloride tablets and metformin hydrochloride extended-release tablets (see "What are the side effects of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?").
Who should not take metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Do not take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets if you:
Can metformin hydrochloride tablets or metformin hydrochloride extended-release tablets be used in children?
How should I take metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
Metformin hydrochloride extended-release tablets must be swallowed whole and never crushed or chewed.
What should I avoid while taking metformin hydrochloride tablets or metformin hydrochloride extended-release tablets?
What are the side effects of metformin hydrochloride tablets and metformin hydrochloride extended-release tablets?
Lactic Acidosis. In rare cases, metformin can cause a serious side effect called lactic acidosis. This is caused by a buildup of lactic acid in your blood. This build-up can cause serious damage.Lactic acidosis caused by metformin hydrochloride tablets and metformin hydrochloride extended-release tablets is rare and has occurred mostly in people whose kidneys were not working normally. Lactic acidosis has been reported in about one in 33,000 patients taking metformin hydrochloride tablets over the course of a year. Although rare, if lactic acidosis does occur, it can be fatal in up to half the people who develop it.
stop using metformin hydrochloride tablets or metformin hydrochloride extended-release tablets and call your doctor right away if you have signs of lactic acidosis. Lactic acidosis is a medical emergency that must be treated in a hospital.
Signs of lactic acidosis are:
General advice about prescription medicines
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Metformin HydrochlorideMETFORMIN HYDROCHLORIDE TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!