Medroxyprogesterone Acetate
FULL PRESCRIBING INFORMATION: CONTENTS*
- BOXED WARNING
- MEDROXYPROGESTERONE ACETATE DESCRIPTION
- CLINICAL PHARMACOLOGY
- PHARMACOKINETICS
- CLINICAL STUDIES
- INDICATIONS & USAGE
- MEDROXYPROGESTERONE ACETATE CONTRAINDICATIONS
- WARNINGS
- PRECAUTIONS
- B. INFORMATION FOR PATIENTS
- C. DRUG & OR LABORATORY TEST INTERACTIONS
- D. CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
- E. PREGNANCY
- F. NURSING MOTHERS
- G. PEDIATRIC USE
- H. GERIATRIC USE
- MEDROXYPROGESTERONE ACETATE ADVERSE REACTIONS
- OVERDOSAGE
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
- SPL PATIENT PACKAGE INSERT
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION
FULL PRESCRIBING INFORMATION
BOXED WARNING
WARNINGSCLINICAL STUDIESWARNINGS, Cardiovascular disordersDementia
CLINICAL STUDIESWARNINGS, Cardiovascular disordersMalignant neoplasmsBreast cancer
CLINICAL STUDIESWARNINGS, DementiaPRECAUTIONS, Geriatric Use
MEDROXYPROGESTERONE ACETATE DESCRIPTION
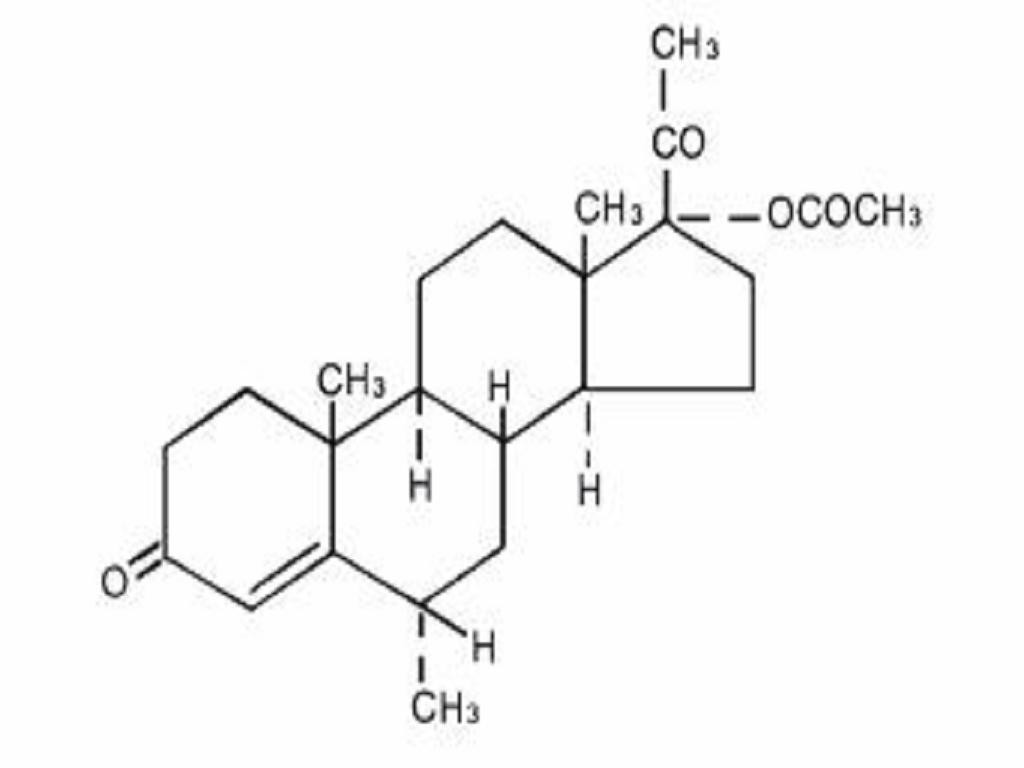
CLINICAL PHARMACOLOGY
PHARMACOKINETICS
Tablet StrengthC max (ng/mL)T max (h)Auc 0((ngt 1/2 (h)Vd/f (L)CL/f (mL/min)**
A. Absorption
B. Distribution
C. Metabolism
D. Excretion
E. Special Populations
Renal Insufficiency
Hepatic Insufficiency
F. Drug Interactions
CLINICAL STUDIES
Effects on the EndometriumTable 2
*
Histological ResultsPlacebo (n=119)CEE(n=119)MPA+ CEE (n=118)*Table 3
CEE*MPA+ CEE* (n=283)MPA 5 mg (n=277)MPA 10 mg (n=272)*
Women's Health Initiative Studies
BOXED WARNINGSWARNINGSPRECAUTIONS
*
EventRelative Risk CE/MPA vs placebo (95%nCI)Placebo n = 8102CE/MPA n = 8506Absolute Risk per 10,000 Women-Years*1.18).Includes metastatic and non-metastatic breast cancer with the exception of in situ breast cancerNominal confidence intervals unadjusted for multiple looks and multiple comparisonsCHD events1.24 (1.001.54)3339Non-fatal MI1.28 (1.001.63)2531CHD death1.10 (0.701.75)88All strokes1.31 (1.021.68)2431Ischemic stroke1.44 (1.091.90)1826Deep vein thrombosis1.95 (1.432.67)1326Pulmonary embolism2.13 (1.453.11)818Invasive breast cancer1.24 (1.011.54)3341Invasive colorectal cancer0.56 (0.380.81)169Endometrial cancer0.81 (0.481.36)76Cervical Cancer1.44 (0.474.42)12Hip fracture0.67 (0.470.96)1611Vertebral fractures0.65 (0.460.92)1711Lower arm/wrist fractures0.71 (0.590.85)6244Total fractures0.76 (0.690.83)199152
Women's Health Initiative Memory Study
The estrogen plus progestin Women's Health Initiative Memory Study (WHIMS), a substudy of WHI, enrolled 4,532 predominantly healthy postmenopausal women 65 years of age and older (47 percent were aged 65 to 69 years, 35 percent were 70 to 74 years, and 18 percent were 75 years of age and older) to evaluate the effects of daily conjugated estrogens (CE 0.625 mg) plus medroxyprogesterone acetate (MPA 2.5 mg) on the incidence of probable dementia (primary outcome) compared with placebo.
After an average follow-up of 4 years, 40 women in the estrogen plus progestin group (45 per 10,000 women-years) and 21 in the placebo group (22 per 10,000 women-years) were diagnosed with probable dementia. The relative risk of probable dementia in the hormone therapy group was 2.05 (95 percent CI, 1.213.48) compared to placebo.
INDICATIONS & USAGE
MEDROXYPROGESTERONE ACETATE CONTRAINDICATIONS
WARNINGS
BOXED WARNINGS1. Cardiovascular disorders
a. Stroke
CLINICAL STUDIES
b. Coronary heart disease
CLINICAL STUDIES
c. Venous thromboembolism (VTE)
CLINICAL STUDIES
2. Malignant neoplasms
a. Breast cancer
CLINICAL STUDIES
b. Endometrial cancer
c. Ovarian cancer
3. Dementia
BOXED WARNINGSPRECAUTIONS, Geriatric Use
4. Visual Abnormalities
PRECAUTIONS
A .General1. Addition of a progestin when a woman has not had a hysterectomy
2. Undiagnosed abnormal vaginal bleeding
3. Elevated blood pressure
4. Hypertriglyceridemia
5. Impaired liver function and past history of cholestatic jaundice
6. Fluid Retention
7. Hypocalcemia
8. Exacerbation of other conditions
B. INFORMATION FOR PATIENTS
C. DRUG & OR LABORATORY TEST INTERACTIONS
D. CARCINOGENESIS & MUTAGENESIS & IMPAIRMENT OF FERTILITY
WARNINGSPRECAUTIONS
E. PREGNANCY
Pregnancy Category XCONTRAINDICATIONS
F. NURSING MOTHERS
G. PEDIATRIC USE
H. GERIATRIC USE
BOXED WARNINGSWARNINGS, Dementia
MEDROXYPROGESTERONE ACETATE ADVERSE REACTIONS
BOXED WARNINGSWARNINGSPRECAUTIONSOVERDOSAGE
DOSAGE & ADMINISTRATION
Secondary AmenorrheaAbnormal Uterine Bleeding Due to Hormonal Imbalance in the Absence of Organic Pathology
Reduction of Endometrial Hyperplasia in Postmenopausal Women Receiving Daily 0.625 mg Conjugated Estrogens
WARNINGS
HOW SUPPLIED
SPL PATIENT PACKAGE INSERT
-
● Treat menstrual periods that have stopped or to treat abnormal uterine bleeding. Women with a uterus who are not pregnant, who stop having regular menstrual periods or who begin to have irregular menstrual periods may have a drop in their progesterone level. Talk with your health care provider about whether medroxyprogesterone acetate tablets are right for you.
-
● Reduce your chances of getting cancer of the uterus. In postmenopausal women with a uterus who use estrogens, taking progestin in combination with estrogen will reduce your chances of getting cancer of the uterus.
-
● Have undiagnosed vaginal bleeding
-
● Currently have or have had certain cancers.
-
● Had a stroke or heart attack in the past year
-
● Currently have or have had blood clots
-
● Currently have or have had liver problems
-
● Think you may be pregnant
-
● Are allergic to any of the ingredients in medroxyprogesterone acetate tablets
-
● If you are breastfeeding. The hormone in medroxyprogesterone acetate tablets can pass into your breast milk.
-
● About all of your medical problems. Your health care provider may need to check you more carefully if you have certain conditions, such as asthma (wheezing); epilepsy (seizures); migraine headaches; endometriosis (severe pelvic pain); lupus; problems with your heart, liver, thyroid, or kidneys; or if you have high calcium levels in your blood.
-
● About all the medicines you take. This includes prescription and nonprescription medicines, vitamins, and herbal supplements. Some medicines may affect how medroxyprogesterone acetate tablets work. Medroxyprogesterone acetate tablets may also affect how other medicines work.
-
● If you are going to have surgery or will be on bed rest. If you are taking estrogen in addition to medroxyprogesterone acetate tablets, you may need to stop taking estrogen and medroxyprogesterone acetate tablets.
-
● Breast tenderness
-
● Breast milk secretion
-
● Breakthrough bleeding
-
● Spotting (minor vaginal bleeding)
-
● Irregular periods
-
● Amenorrhea (absence of menstrual periods)
-
● Vaginal secretions
-
● Headaches
-
● Nervousness
-
● Dizziness
-
● Depression
-
● Insomnia, sleepiness, fatigue
-
● Premenstrual syndrome-like symptoms
-
● Thrombophlebitis (inflamed veins)
-
● Blood clot
-
● Itching, hives, skin rash
-
● Acne
-
● Hair loss, hair growth
-
● Abdominal discomfort
-
● Nausea
-
● Bloating
-
● Fever
-
● Increase in weight
-
● Swelling
-
● Changes in vision and sensitivity to contact lenses
-
● Breast cancer
-
● Cancer of the uterus
-
● Stroke
-
● Heart attack
-
● Blood clots
-
● Dementia
-
● Gallbladder disease
-
● Ovarian cancer
-
● High blood pressure
-
● Liver problems
-
● High blood sugar
-
● Enlargement of benign tumors of the uterus ("fibroids")
-
● Breast lumps
-
● Unusual vaginal bleeding
-
● Dizziness and faintness
-
● Changes in speech
-
● Severe headaches
-
● Chest pain
-
● Shortness of breath
-
● Pains in your legs
-
● Changes in vision
-
● Vomiting
-
● Yellowing of the skin, eyes or nail beds
-
● Headache
-
● Breast pain
-
● Irregular vaginal bleeding or spotting
-
● Stomach/abdominal cramps, bloating
-
● Nausea and vomiting
-
● Hair loss
-
● Fluid retention
-
● Vaginal yeast infection
-
● Talk with your health care provider regularly about whether you should continue taking medroxyprogesterone acetate tablets. The addition of a progestin is generally recommended for women with a uterus to reduce the chance of getting cancer of the uterus.
-
● See your health care provider right away if you get vaginal bleeding while taking medroxyprogesterone acetate tablets.
-
● Have a pelvic exam, breast exam and mammogram (breast X-ray) every year unless your health care provider tells you otherwise. If members of your family have had breast cancer or if you have ever had breast lumps or an abnormal mammogram, you may need to have breast exams more often.
-
● If you have high blood pressure, high cholesterol (fat in the blood), diabetes, are overweight, or if you use tobacco, you may have a higher chance of getting heart disease. Ask your health care provider for ways to lower your chance of getting heart disease.
-
● Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets.
-
● Do not take medroxyprogesterone acetate tablets for conditions for which it was not prescribed.
-
● Do not give medroxyprogesterone acetate tablets to other people, even if they have the same symptoms you have. It may harm them.
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL SECTION

Medroxyprogesterone AcetateMedroxyprogesterone Acetate TABLET
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PLEASE, BE CAREFUL!
Be sure to consult your doctor before taking any medication!